Ann Lab Med.
2024 Nov;44(6):576-580. 10.3343/alm.2024.0086.
Association Between Clonal Hematopoiesis of Indeterminate Potential and Brain β-Amyloid Deposition in Korean Patients With Cognitive Impairment
- Affiliations
-
- 1Department of Laboratory Medicine, Korea University College of Medicine, Seoul, Korea
- 2Department of Neurology, Chung-Ang University College of Medicine, Seoul, Korea
- 3Department of Laboratory Medicine, Chung-Ang University College of Medicine, Seoul, Korea
- KMID: 2560805
- DOI: http://doi.org/10.3343/alm.2024.0086
Abstract
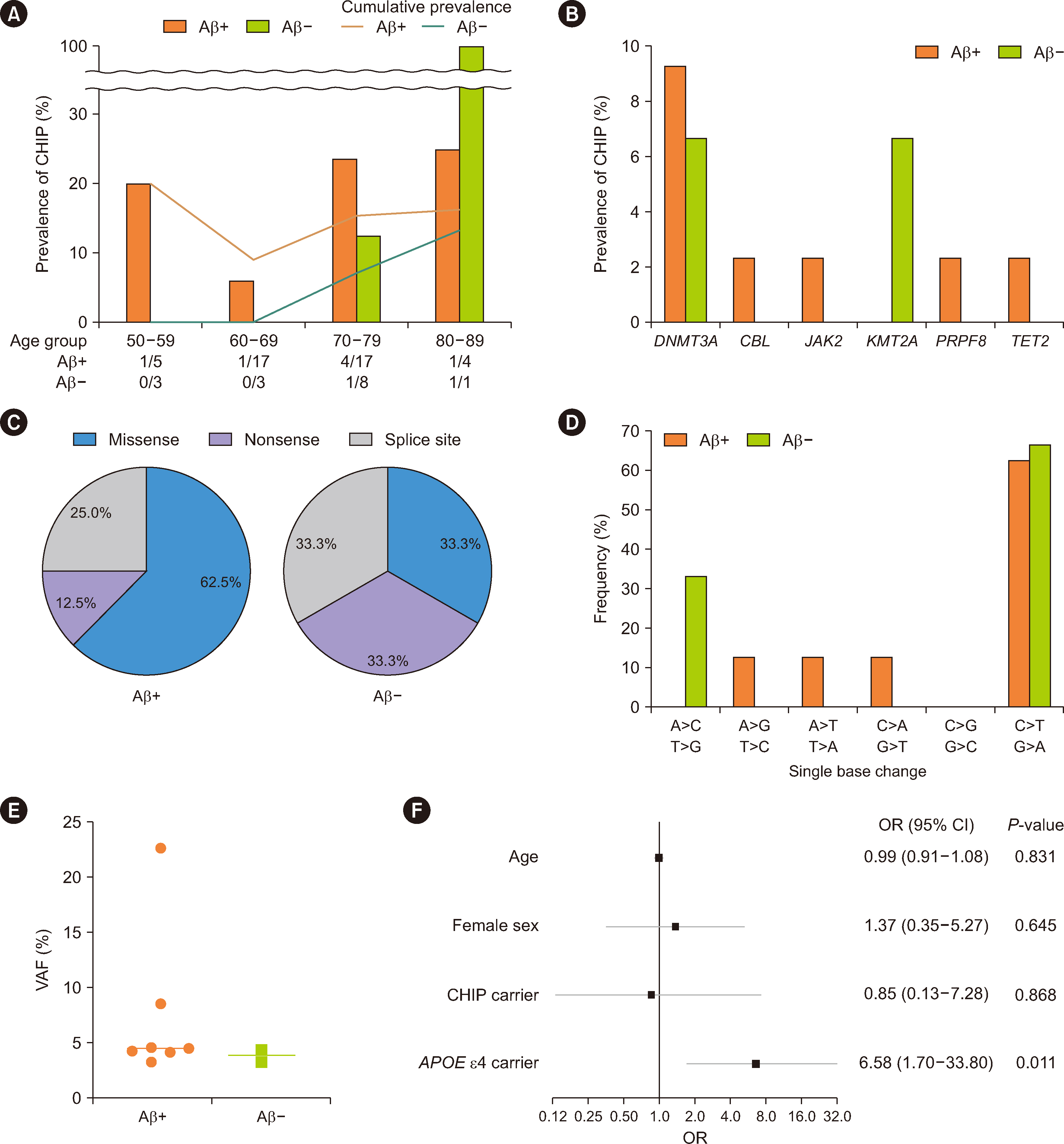
- Few studies have focused on the association between clonal hematopoiesis of indeterminate potential (CHIP) and β-amyloid (Aβ) deposition in the brain, which causes Alzheimer’s disease. We aimed to investigate the potential role of CHIP in brain Aβ deposition in Korean patients. We enrolled 58 Korean patients over 50 yrs of age with cognitive impairment who underwent brain Aβ positron emission tomography. We explored CHIP in their peripheral blood using deep-targeted next-generation sequencing. Irrespective of the presence or absence of brain Aβ deposition, mutations in DNMT3A and the C:G > T:A single-nucleotide variants were identified as the primary characteristics, which reflect aged hematopoiesis in the study population. Multivariate logistic regression revealed that the presence of CHIP was not associated with brain Aβ deposition. As both CHIP and brain Aβ deposition are associated with aging, further research is required to elucidate their possible interplay.
Keyword
Figure
Reference
-
References
1. Steensma DP, Bejar R, Jaiswal S, Lindsley RC, Sekeres MA, Hasserjian RP, et al. 2015; Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood. 126:9–16. DOI: 10.1182/blood-2015-03-631747. PMID: 25931582. PMCID: PMC4624443.2. Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, et al. 2014; Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 371:2488–98. DOI: 10.1056/NEJMoa1408617. PMID: 25426837. PMCID: PMC4306669.3. Jaiswal S, Libby P. 2020; Clonal haematopoiesis: connecting ageing and inflammation in cardiovascular disease. Nat Rev Cardiol. 17:137–44. DOI: 10.1038/s41569-019-0247-5. PMID: 31406340. PMCID: PMC9448847.4. Kim M, Kim JJ, Lee ST, Shim Y, Lee H, Bae S, et al. 2024; Association between aortic valve sclerosis and clonal hematopoiesis of indeterminate potential. Ann Lab Med. 44:279–88. DOI: 10.3343/alm.2023.0268. PMID: 38205526. PMCID: PMC10813825.5. Mas-Peiro S, Hoffmann J, Fichtlscherer S, Dorsheimer L, Rieger MA, Dimmeler S, et al. 2020; Clonal haematopoiesis in patients with degenerative aortic valve stenosis undergoing transcatheter aortic valve implantation. Eur Heart J. 41:933–9. DOI: 10.1093/eurheartj/ehz591. PMID: 31504400. PMCID: PMC7033916.6. Dorsheimer L, Assmus B, Rasper T, Ortmann CA, Ecke A, Abou-El-Ardat K, et al. 2019; Association of mutations contributing to clonal hematopoiesis with prognosis in chronic ischemic heart failure. JAMA Cardiol. 4:25–33. DOI: 10.1001/jamacardio.2018.3965. PMID: 30566180. PMCID: PMC6439691.7. Buscarlet M, Provost S, Zada YF, Barhdadi A, Bourgoin V, Lépine G, et al. 2017; DNMT3A and TET2 dominate clonal hematopoiesis and demonstrate benign phenotypes and different genetic predispositions. Blood. 130:753–62. DOI: 10.1182/blood-2017-04-777029. PMID: 28655780.8. Zink F, Stacey SN, Norddahl GL, Frigge ML, Magnusson OT, Jonsdottir I, et al. 2017; Clonal hematopoiesis, with and without candidate driver mutations, is common in the elderly. Blood. 130:742–52. DOI: 10.1182/blood-2017-02-769869. PMID: 28483762. PMCID: PMC5553576.9. Savola P, Lundgren S, Keränen MAI, Almusa H, Ellonen P, Leirisalo-Repo M, et al. 2018; Clonal hematopoiesis in patients with rheumatoid arthritis. Blood Cancer J. 8:69. DOI: 10.1038/s41408-018-0107-2. PMID: 30061683. PMCID: PMC6066480.10. Bouzid H, Belk JA, Jan M, Qi Y, Sarnowski C, Wirth S, et al. 2023; Clonal hematopoiesis is associated with protection from Alzheimer's disease. Nat Med. 29:1662–70. DOI: 10.1038/s41591-023-02397-2. PMID: 37322115. PMCID: PMC10353941.11. U.S. Food & Drug Administration. Drugs@FDA: FDA-approved drugs. Amyvid/labels for NDA202008. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202008s000lbl.pdf. Updated on April 2012.12. U.S. Food & Drug Administration. Drugs@FDA: FDA-approved drugs. Neuraceq/labels for NDA204677. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/204677s000lbl.pdf. Updated on March 2014.13. U.S. Food & Drug Administration. Drugs@FDA: FDA-approved drugs. Vizamyl/labels for NDA203137. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/203137s000lbl.pdf. Updated on October 2013.14. Sabri O, Seibyl J, Rowe C, Barthel H. 2015; Beta-amyloid imaging with florbetaben. Clin Transl Imaging. 3:13–26. DOI: 10.1007/s40336-015-0102-6. PMID: 25741488. PMCID: PMC4339690.15. Jessen F, Amariglio RE, van Boxtel M, Breteler M, Ceccaldi M, Chételat G, et al. 2014; A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimers Dement. 10:844–52. DOI: 10.1016/j.jalz.2014.01.001. PMID: 24798886. PMCID: PMC4317324.16. Petersen RC. 2011; Clinical practice. Mild cognitive impairment. N Engl J Med. 364:2227–34. DOI: 10.1056/NEJMcp0910237. PMID: 21651394.17. American Psychiatric Association. 2022. Diagnostic and statistical manual of mental disorders. 5th ed., text revision. American Psychiatric Association;Washington, DC: DOI: 10.1176/appi.books.9780890425787.18. Bolton KL, Koh Y, Foote MB, Im H, Jee J, Sun CH, et al. 2021; Clonal hematopoiesis is associated with risk of severe Covid-19. Nat Commun. 12:5975. DOI: 10.1038/s41467-021-26138-6. PMID: 34645798. PMCID: PMC8514469. PMID: 3cb397d07d014cc5b6487816e9888d83.19. Moon I, Kong MG, Ji YS, Kim SH, Park SK, Suh J, et al. 2023; Clinical, mutational, and transcriptomic characteristics in elderly Korean individuals with clonal hematopoiesis driver mutations. Ann Lab Med. 43:145–52. DOI: 10.3343/alm.2023.43.2.145. PMID: 36281508. PMCID: PMC9618905.20. Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, et al. 2013; Signatures of mutational processes in human cancer. Nature. 500:415–21. DOI: 10.1038/nature12477. PMID: 23945592. PMCID: PMC3776390.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current Aspects of Clonal Hematopoiesis: Implications for Clinical Diagnosis
- Asymmetric Amyloid-β Burden and Neurodegeneration in Late-Onset Alzheimer’s Disease Dementia
- Comparison of Amyloid Positivity Rate and Accumulation Pattern between Amnestic and Non-Amnestic Type Mild Cognitive Impairment
- Contributing Factors to Diabetic Brain Injury and Cognitive Decline
- Association between Cerebral Amyloid Deposition and Clinical Factors Including Cognitive Function in Geriatric Depression: Pilot Study Using Amyloid Positron Emission Tomography