Endocrinol Metab.
2022 Apr;37(2):243-248. 10.3803/EnM.2021.1337.
The Presence of Clonal Hematopoiesis Is Negatively Associated with Diabetic Peripheral Neuropathy in Type 2 Diabetes
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea
- 2Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea
- 3Genome Opinion Incorporation, Seoul, Korea
- 4Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea
- KMID: 2529216
- DOI: http://doi.org/10.3803/EnM.2021.1337
Abstract
- Background
Clonal hematopoiesis of indeterminate potential (CHIP) has been reported to be associated with increased cardiovascular disease, aging and insulin resistance. Despite the debate of causal contribution of CHIP on metabolic diseases, we want to explore whether CHIP is related to diabetic peripheral neuropathy (DPN).
Methods
This study analyzed the prevalence of CHIP in patients with type 2 diabetes classified according to DPN status. Logistic regression analysis was used to evaluate the association between CHIP and DPN.
Results
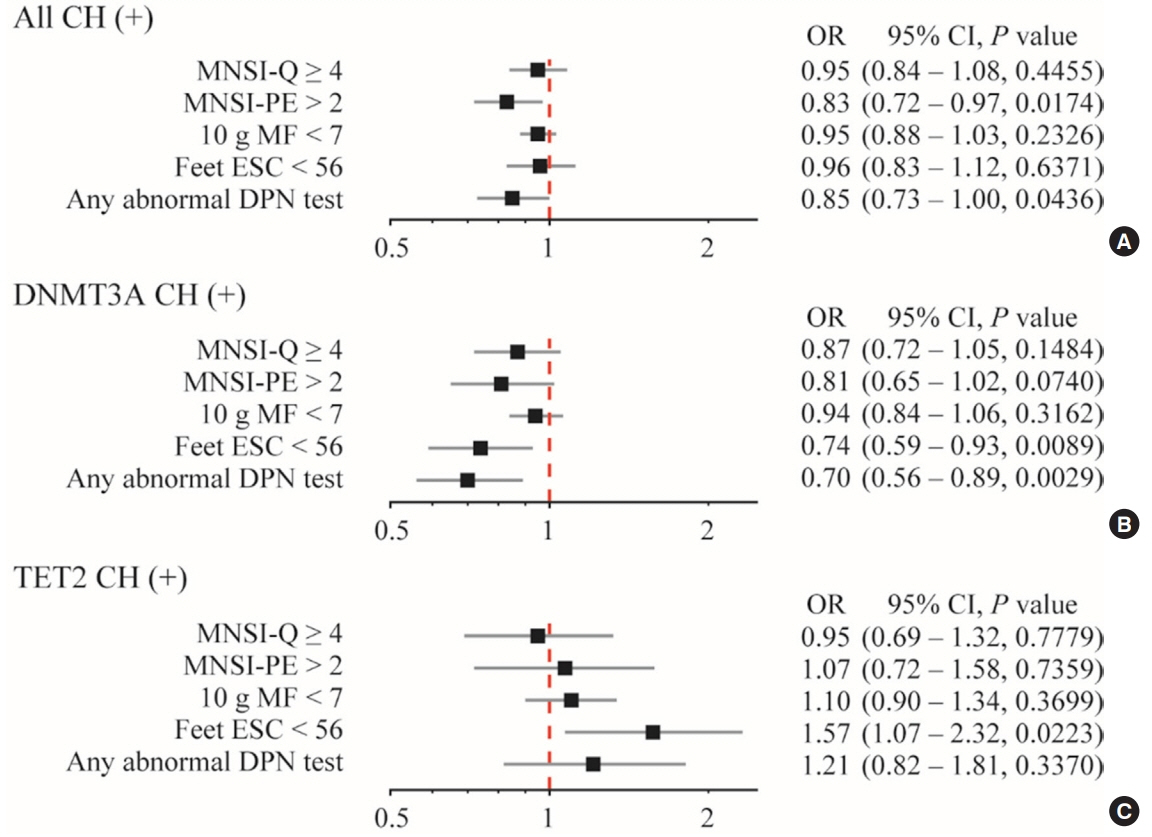
CHIP was more prevalent in subjects without DPN than those with DPN (19.9% vs. 8.8%, respectively; P=0.013). Individuals having any CHIP, or DNA methyltransferase 3A (DNMT3A) CHIP were less likely to have any abnormality shown in DPN test; the adjusted odds ratio were 0.85 (95% confidence interval [CI], 0.73 to 1.00) and 0.70 (95% CI, 0.56 to 0.89), respectively. Interestingly, DNMT3A CHIP showed the negative association, but Tet methylcytosine dioxygenase 2 (TET2) CHIP showed the positive association with abnormal feet electrochemical skin conductance level.
Conclusion
On the contrary to expectations, CHIP was negatively associated with DPN. Functional linking between the mutation in hematopoietic cells and DPN, and the opposite role of DNMT3A and TET2 should be investigated.
Figure
Reference
-
1. Jaiswal S, Ebert BL. Clonal hematopoiesis in human aging and disease. Science. 2019; 366:eaan4673.
Article2. Coombs CC, Zehir A, Devlin SM, Kishtagari A, Syed A, Jonsson P, et al. Therapy-related clonal hematopoiesis in patients with non-hematologic cancers is common and associated with adverse clinical outcomes. Cell Stem Cell. 2017; 21:374–82.
Article3. Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014; 371:2488–98.
Article4. Tesfaye S, Chaturvedi N, Eaton SE, Ward JD, Manes C, Ionescu-Tirgoviste C, et al. Vascular risk factors and diabetic neuropathy. N Engl J Med. 2005; 352:341–50.
Article5. Chung JO, Cho DH, Chung DJ, Chung MY. Association between diabetic polyneuropathy and cardiovascular complications in type 2 diabetic patients. Diabetes Metab J. 2011; 35:390–6.
Article6. Roustit M, Loader J, Deusenbery C, Baltzis D, Veves A. Endothelial dysfunction as a link between cardiovascular risk factors and peripheral neuropathy in diabetes. J Clin Endocrinol Metab. 2016; 101:3401–8.
Article7. Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care. 1994; 17:1281–9.
Article8. Won JC, Park TS. Recent advances in diagnostic strategies for diabetic peripheral neuropathy. Endocrinol Metab (Seoul). 2016; 31:230–8.
Article9. Oh TJ, Song Y, Jang HC, Choi SH. SUDOSCAN in combination with the Michigan neuropathy screening instrument is an effective tool for screening diabetic peripheral neuropathy. Diabetes Metab J. 2022; 46:319–26.
Article10. Bolton KL, Koh Y, Foote MB, Im H, Jee J, Sun CH, et al. Clonal hematopoiesis is associated with risk of severe Covid-19. medRxiv. 2020; Nov. 27. [Preprint]. https://doi.org/10.1101/2020.11.25.20233163.
Article11. Honigberg MC, Zekavat SM, Niroula A, Griffin GK, Bick AG, Pirruccello JP, et al. Premature menopause, clonal hematopoiesis, and coronary artery disease in postmenopausal women. Circulation. 2021; 143:410–23.
Article12. Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, et al. Clonal Hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med. 2017; 377:111–21.
Article13. Schubeler D. Function and information content of DNA methylation. Nature. 2015; 517:321–6.
Article14. Buscarlet M, Provost S, Zada YF, Bourgoin V, Mollica L, Dube MP, et al. Lineage restriction analyses in CHIP indicate myeloid bias for TET2 and multipotent stem cell origin for DNMT3A. Blood. 2018; 132:277–80.
Article15. Fuster JJ, Zuriaga MA, Zorita V, MacLauchlan S, Polackal MN, Viana-Huete V, et al. TET2-loss-of-function-driven clonal hematopoiesis exacerbates experimental insulin resistance in aging and obesity. Cell Rep. 2020; 33:108326.
Article16. Weng YL, Joseph J, An R, Song H, Ming GL. Epigenetic regulation of axonal regenerative capacity. Epigenomics. 2016; 8:1429–42.
Article17. Shao C, Gao Y, Jin D, Xu X, Tan S, Yu H, et al. DNMT3a methylation in neuropathic pain. J Pain Res. 2017; 10:2253–62.
Article18. Li T, Yang D, Li J, Tang Y, Yang J, Le W. Critical role of Tet3 in neural progenitor cell maintenance and terminal differentiation. Mol Neurobiol. 2015; 51:142–54.
Article19. Selvarajah D, Cash T, Davies J, Sankar A, Rao G, Grieg M, et al. SUDOSCAN: a simple, rapid, and objective method with potential for screening for diabetic peripheral neuropathy. PLoS One. 2015; 10:e0138224.
Article20. Petropoulos IN, Ponirakis G, Khan A, Almuhannadi H, Gad H, Malik RA. Diagnosing diabetic neuropathy: something old, something new. Diabetes Metab J. 2018; 42:255–69.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diabetic Peripheral Neuropathy in Type 2 Diabetes Mellitus in Korea
- Computerized Dynamic Posturography and Diabetic Peripheral Neuropathy
- Diabetic Neuropathy
- Erythropoietin Levels According to the Presence of Peripheral Neuropathy in Diabetic Patients with Anemia
- Pathogenesis of the Diabetic Foot Disease