J Yeungnam Med Sci.
2024 Apr;41(2):103-112. 10.12701/jyms.2023.01389.
DA-6034 ameliorates hepatic steatosis and inflammation in high fat diet-induced obese mice
- Affiliations
-
- 1Astrogen Inc., Daegu, Korea
- 2The East Coast Research Institute of Life Science, Gangneung-Wonju National University, Gangneung, Korea
- 3Department of Internal Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea
- 4Research Institute of Metabolism and Inflammation, Yonsei University Wonju College of Medicine, Wonju, Korea
- 5Department of Clinical Research, Vaccine Center for Assisting Safety & Technology, Hwasun, Korea
- KMID: 2554771
- DOI: http://doi.org/10.12701/jyms.2023.01389
Abstract
- Background
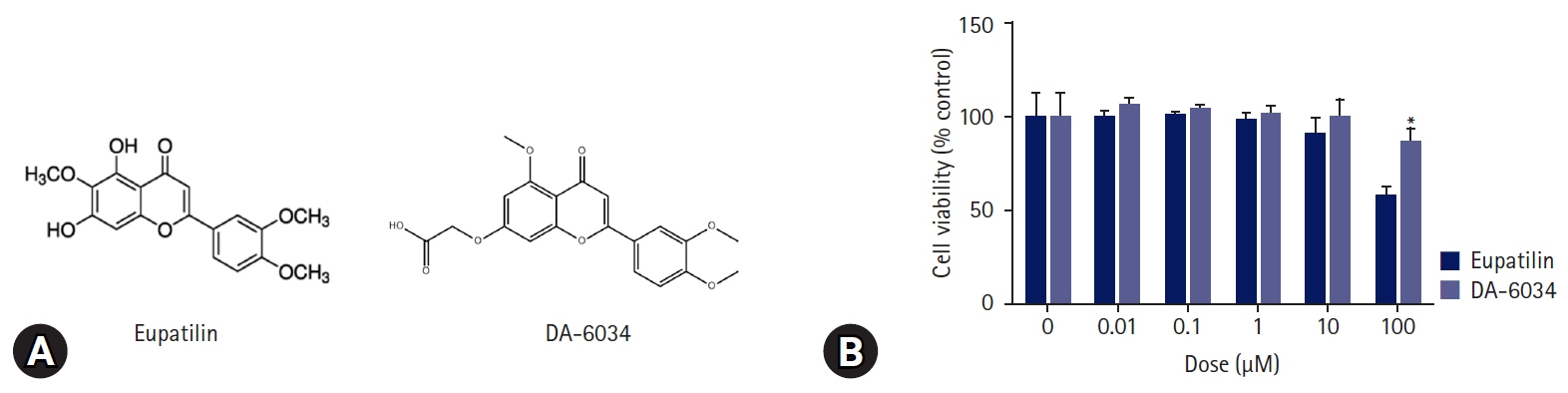
Nonalcoholic fatty liver disease (NAFLD) is characterized by an increase in hepatic triglyceride content and increased inflammatory macrophage infiltration through the C-C motif chemokine receptor (CCR) 5 pathway in the liver. DA-6034 (7-carboxymethyloxy-3',4',5-trimethoxy flavone), is a synthetic derivative of eupatilin that exhibits anti-inflammatory activity in inflammatory bowel disease. However, the effect of DA-6034 on the inflammatory response in NAFLD is not well elucidated. Therefore, we aimed to determine the effect of DA-6034 on hepatic steatosis and inflammation.
Methods
Forty male C57BL/6J mice were divided into the following four groups: (1) regular diet (RD), (2) RD with DA-6034, (3) high fat diet (HFD), and (4) HFD with DA-6034. All mice were sacrificed 12 weeks after the start of the experiment. The effects of DA-6034 on macrophages were assessed using RAW264.7 cells.
Results
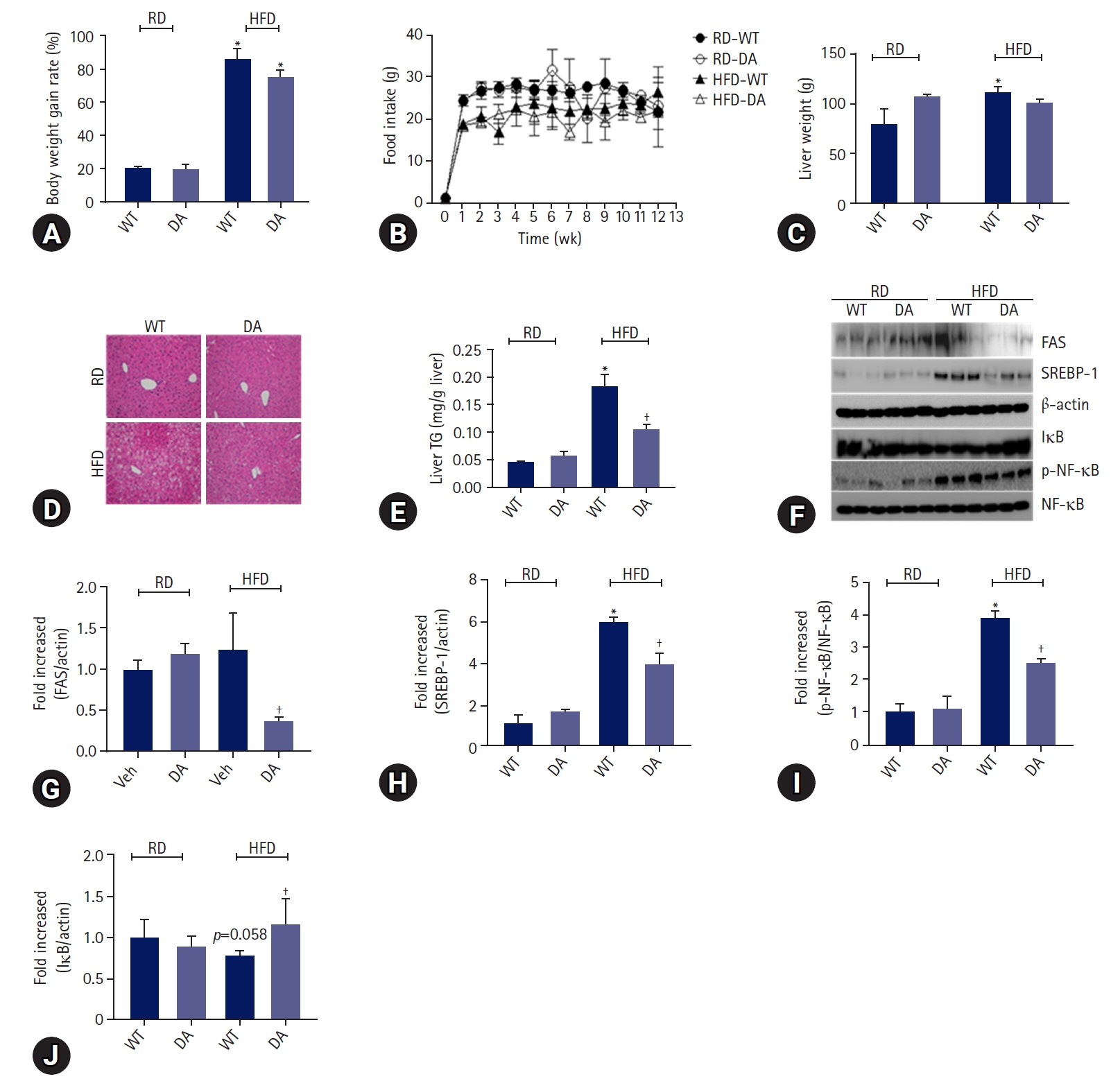
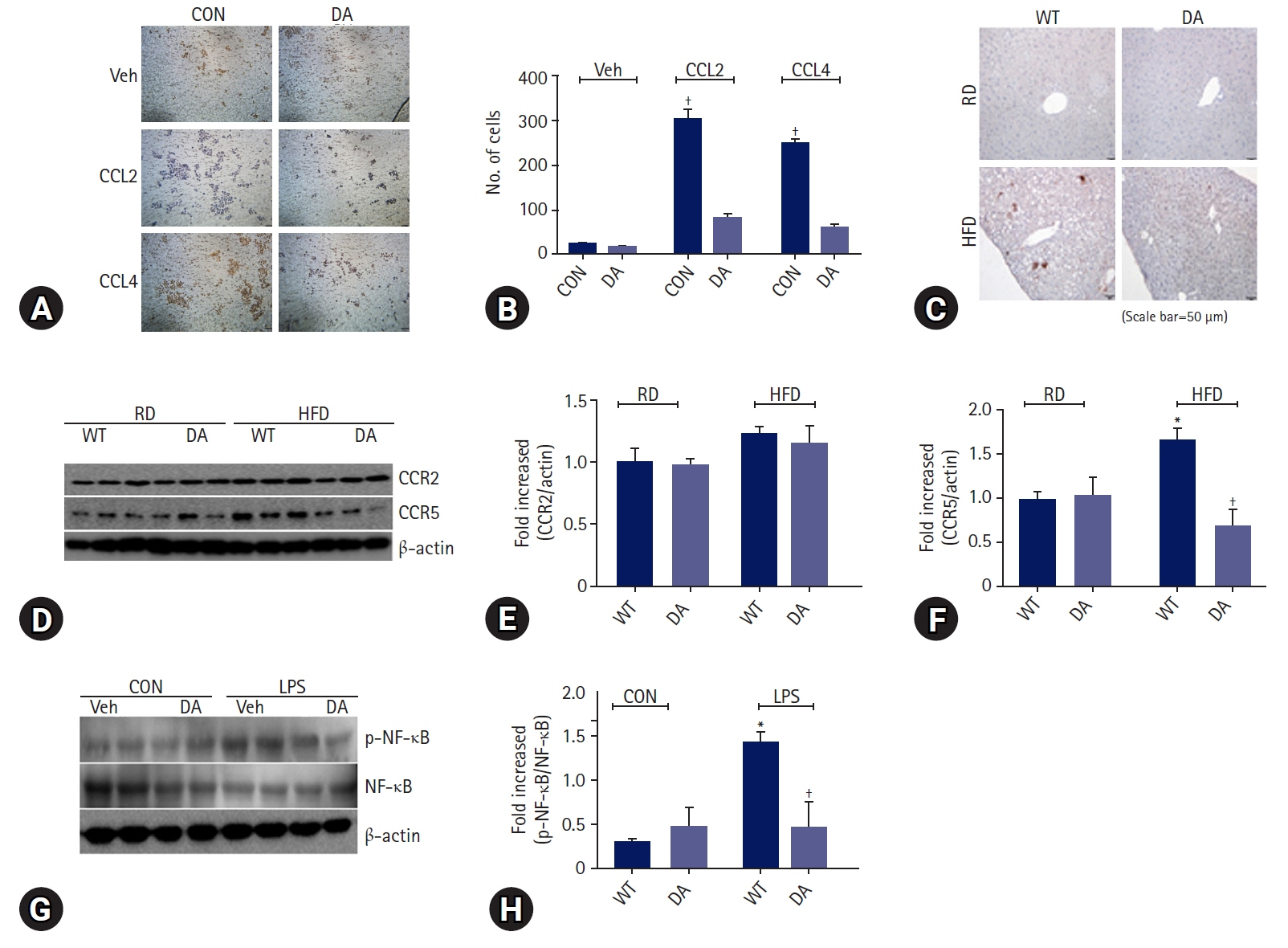
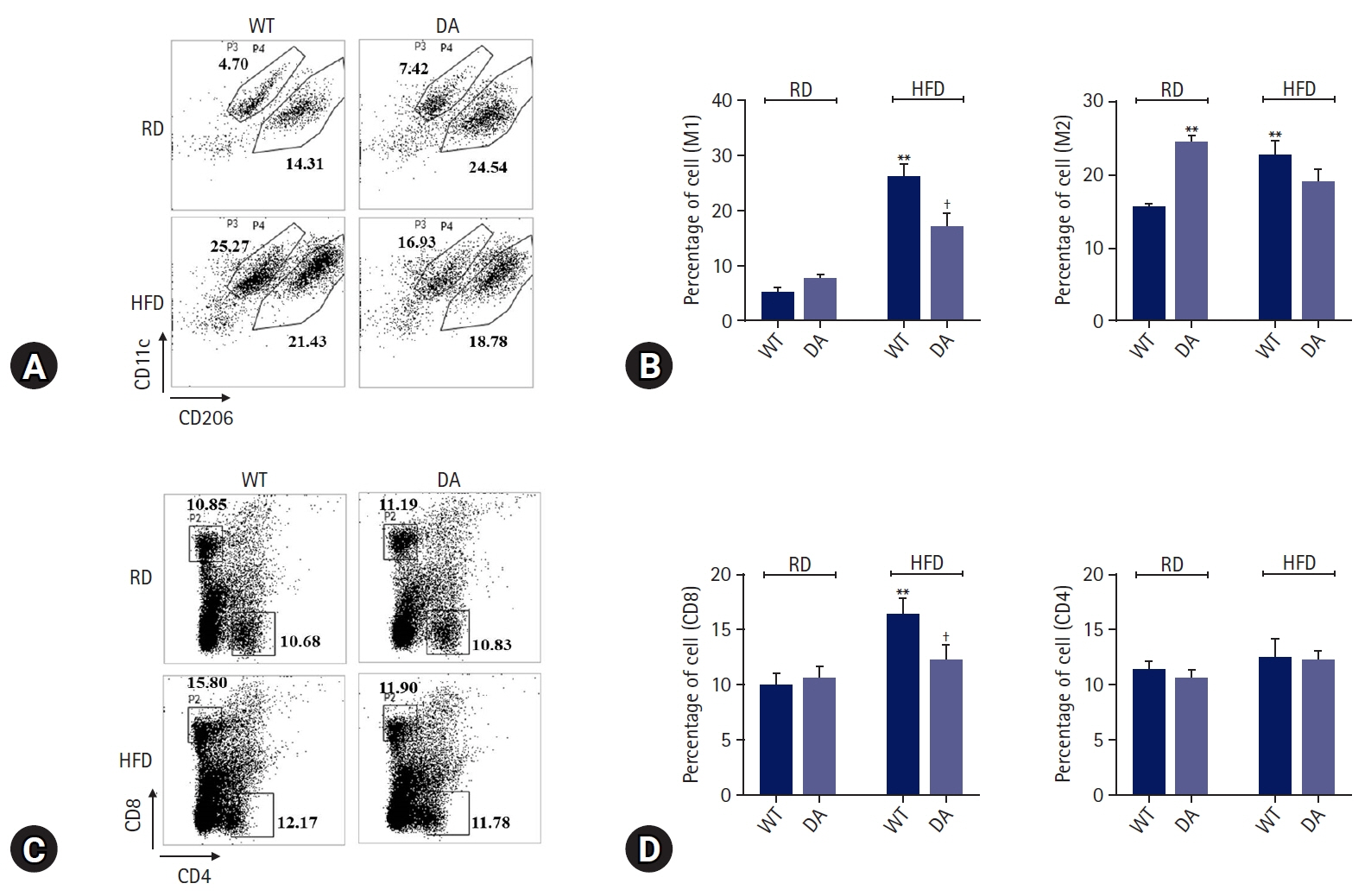
DA-6034 not only reduced hepatic triglyceride levels and lipid accumulation but also macrophage infiltration and proinflammatory cytokines in HFD-fed mice. According to fluorescence-activated cell sorter analysis, DA-6034 reduced the CD8+ T cell fraction in the liver of HFD-fed mice. DA-6034 also reduced CCR5 expression and the migration of liver macrophages in HFD-fed mice and inhibited CCR2 ligand and CCR4 ligand, which stimulated the migration of macrophages.
Conclusion
Overall, DA-6034 attenuates hepatic steatosis and inflammation in obesity by regulating CCR5 expression in macrophages.
Keyword
Figure
Reference
-
References
1. Tiniakos DG, Vos MB, Brunt EM. Nonalcoholic fatty liver disease: pathology and pathogenesis. Annu Rev Pathol. 2010; 5:145–71.
Article2. Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011; 332:1519–23.
Article3. Koyama Y, Brenner DA. Liver inflammation and fibrosis. J Clin Invest. 2017; 127:55–64.
Article4. Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017; 2:17023.
Article5. Baker RG, Hayden MS, Ghosh S. NF-κB, inflammation, and metabolic disease. Cell Metab. 2011; 13:11–22.
Article6. Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 2001; 107:7–11.7. Lee BC, Lee J. Cellular and molecular players in adipose tissue inflammation in the development of obesity-induced insulin resistance. Biochim Biophys Acta. 2014; 1842:446–62.
Article8. Monteiro R, Azevedo I. Chronic inflammation in obesity and the metabolic syndrome. Mediators Inflamm. 2010; 2010:289645.
Article9. Fei X, Chen C, Kai S, Fu X, Man W, Ding B, et al. Eupatilin attenuates the inflammatory response induced by intracerebral hemorrhage through the TLR4/MyD88 pathway. Int Immunopharmacol. 2019; 76:105837.
Article10. Du L, Chen J, Xing YQ. Eupatilin prevents H2O2-induced oxidative stress and apoptosis in human retinal pigment epithelial cells. Biomed Pharmacother. 2017; 85:136–40.
Article11. Zhong WF, Wang XH, Pan B, Li F, Kuang L, Su ZX. Eupatilin induces human renal cancer cell apoptosis via ROS-mediated MAPK and PI3K/AKT signaling pathways. Oncol Lett. 2016; 12:2894–9.
Article12. Lee S, Lee M, Kim SH. Eupatilin inhibits H(2)O(2)-induced apoptotic cell death through inhibition of mitogen-activated protein kinases and nuclear factor-kappaB. Food Chem Toxicol. 2008; 46:2865–70.13. Kim YW, Lee WH, Choi SM, Seo YY, Ahn BO, Kim SH, et al. DA6034 promotes gastric epithelial cell migration and wound-healing through the mTOR pathway. J Gastroenterol Hepatol. 2012; 27:397–405.
Article14. Chung HJ, Choi YH, Choi HD, Jang JM, Shim HJ, Yoo M, et al. Pharmacokinetics of DA-6034, an agent for inflammatory bowel disease, in rats and dogs: contribution of intestinal first-pass effect to low bioavailability in rats. Eur J Pharm Sci. 2006; 27:363–74.
Article15. Kim YS, Son M, Ko JI, Cho H, Yoo M, Kim WB, et al. Effect of DA-6034, a derivative of flavonoid, on experimental animal models of inflammatory bowel disease. Arch Pharm Res. 1999; 22:354–60.
Article16. Jump DB, Botolin D, Wang Y, Xu J, Christian B, Demeure O. Fatty acid regulation of hepatic gene transcription. J Nutr. 2005; 135:2503–6.
Article17. Pettinelli P, Obregón AM, Videla LA. Molecular mechanisms of steatosis in nonalcoholic fatty liver disease. Nutr Hosp. 2011; 26:441–50.18. Kuan YC, Hashidume T, Shibata T, Uchida K, Shimizu M, Inoue J, et al. Heat shock protein 90 modulates lipid homeostasis by regulating the stability and function of sterol regulatory element-binding protein (SREBP) and SREBP cleavage-activating protein. J Biol Chem. 2017; 292:3016–28.
Article19. Ko SH, Yoo DY, Kim YJ, Choi SM, Kang KK, Kim H, et al. A mechanism for the action of the compound DA-6034 on NF-κB pathway activation in Helicobacter pylori-infected gastric epithelial cells. Scand J Immunol. 2011; 74:253–63.
Article20. Stienstra R, Netea-Maier RT, Riksen NP, Joosten LA, Netea MG. Specific and complex reprogramming of cellular metabolism in myeloid cells during innate immune responses. Cell Metab. 2017; 26:142–56.
Article21. Reilly SM, Saltiel AR. Adapting to obesity with adipose tissue inflammation. Nat Rev Endocrinol. 2017; 13:633–43.
Article22. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003; 112:1796–808.
Article23. Stienstra R, Saudale F, Duval C, Keshtkar S, Groener JE, van Rooijen N, et al. Kupffer cells promote hepatic steatosis via interleukin-1beta-dependent suppression of peroxisome proliferator-activated receptor alpha activity. Hepatology. 2010; 51:511–22.
Article24. Morgan PK, Huynh K, Pernes G, Miotto PM, Mellett NA, Giles C, et al. Macrophage polarization state affects lipid composition and the channeling of exogenous fatty acids into endogenous lipid pools. J Biol Chem. 2021; 297:101341.
Article25. Castoldi A, Monteiro LB, van Teijlingen Bakker N, Sanin DE, Rana N, Corrado M, et al. Triacylglycerol synthesis enhances macrophage inflammatory function. Nat Commun. 2020; 11:4107.
Article26. Manne V, Handa P, Kowdley KV. Pathophysiology of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Clin Liver Dis. 2018; 22:23–37.
Article27. Maeda S, Chang L, Li ZW, Luo JL, Leffert H, Karin M. IKKbeta is required for prevention of apoptosis mediated by cell-bound but not by circulating TNFalpha. Immunity. 2003; 19:725–37.
Article28. Luedde T, Assmus U, Wüstefeld T, Meyer zu Vilsendorf A, Roskams T, Schmidt-Supprian M, et al. Deletion of IKK2 in hepatocytes does not sensitize these cells to TNF-induced apoptosis but protects from ischemia/reperfusion injury. J Clin Invest. 2005; 115:849–59.
Article29. Luedde T, Schwabe RF. NF-κB in the liver: linking injury, fibrosis and hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2011; 8:108–18.
Article30. Stone MJ, Hayward JA, Huang C, E Huma Z, Sanchez J. Mechanisms of regulation of the chemokine-receptor network. Int J Mol Sci. 2017; 18:342.
Article31. Yang H, Zhang Q, Xu M, Wang L, Chen X, Feng Y, et al. CCL2-CCR2 axis recruits tumor associated macrophages to induce immune evasion through PD-1 signaling in esophageal carcinogenesis. Mol Cancer. 2020; 19:41.
Article32. Parker R, Weston CJ, Miao Z, Corbett C, Armstrong MJ, Ertl L, et al. CC chemokine receptor 2 promotes recruitment of myeloid cells associated with insulin resistance in nonalcoholic fatty liver disease. Am J Physiol Gastrointest Liver Physiol. 2018; 314:G483–93.
Article33. Lee SJ, Kang JS, Kim HM, Lee ES, Lee JH, Chung CH, et al. CCR2 knockout ameliorates obesity-induced kidney injury through inhibiting oxidative stress and ER stress. PLoS One. 2019; 14:e0222352.
Article34. Gutierrez DA, Kennedy A, Orr JS, Anderson EK, Webb CD, Gerrald WK, et al. Aberrant accumulation of undifferentiated myeloid cells in the adipose tissue of CCR2-deficient mice delays improvements in insulin sensitivity. Diabetes. 2011; 60:2820–9.
Article35. Kitade H, Sawamoto K, Nagashimada M, Inoue H, Yamamoto Y, Sai Y, et al. CCR5 plays a critical role in obesity-induced adipose tissue inflammation and insulin resistance by regulating both macrophage recruitment and M1/M2 status. Diabetes. 2012; 61:1680–90.
Article36. Huh JH, Kim HM, Lee ES, Kwon MH, Lee BR, Ko HJ, et al. Dual CCR2/5 antagonist attenuates obesity-induced insulin resistance by regulating macrophage recruitment and M1/M2 status. Obesity (Silver Spring). 2018; 26:378–86.
Article37. Kim HM, Kim YM, Huh JH, Lee ES, Kwon MH, Lee BR, et al. α-Mangostin ameliorates hepatic steatosis and insulin resistance by inhibition C-C chemokine receptor 2. PLoS One. 2017; 12:e0179204.
Article38. Ambade A, Lowe P, Kodys K, Catalano D, Gyongyosi B, Cho Y, et al. Pharmacological inhibition of CCR2/5 signaling prevents and reverses alcohol-induced liver damage, steatosis, and inflammation in mice. Hepatology. 2019; 69:1105–21.
Article39. Tosello-Trampont AC, Landes SG, Nguyen V, Novobrantseva TI, Hahn YS. Kuppfer cells trigger nonalcoholic steatohepatitis development in diet-induced mouse model through tumor necrosis factor-α production. J Biol Chem. 2012; 287:40161–72.
Article40. Han R, Zhang F, Wan C, Liu L, Zhong Q, Ding W. Effect of perfluorooctane sulphonate-induced Kupffer cell activation on hepatocyte proliferation through the NF-κB/TNF-α/IL-6-dependent pathway. Chemosphere. 2018; 200:283–94.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Kaempferol ameliorates metabolic syndrome by inhibiting inflammation and oxidative stress in high-fat diet-induced obese mice
- Umbelliferone Ameliorates Hepatic Steatosis and Lipid-Induced ER Stress in High-Fat Diet-Induced Obese Mice
- Myeloid-specific SIRT1 Deletion Aggravates Hepatic Inflammation and Steatosis in High-fat Diet-fed Mice
- Mentha canadensis attenuates adiposity and hepatic steatosis in high-fat diet-induced obese mice
- Effects of the Combination of Evogliptin and Leucine on Insulin Resistance and Hepatic Steatosis in High-Fat Diet-Fed Mice