Nutr Res Pract.
2023 Oct;17(5):870-882. 10.4162/nrp.2023.17.5.870.
Mentha canadensis attenuates adiposity and hepatic steatosis in high-fat diet-induced obese mice
- Affiliations
-
- 1Biological Clock-based Anti-aging Convergence Regional Leading Research Center of National Research Foundation of Korea, Korea University, Sejong 30019, Korea
- 2Department of Food and Nutrition, Chosun University, Gwangju 61452, Korea
- 3Department of Food Science and Nutrition, Kyungpook National University, Daegu 41566, Korea
- 4Center for Food and Nutritional Genomics Research, Kyungpook National University, Daegu 41566, Korea
- 5Center for Beautiful Aging, Kyungpook National University, Daegu 41566, Korea
- KMID: 2546945
- DOI: http://doi.org/10.4162/nrp.2023.17.5.870
Abstract
- BACKGROUND/OBJECTIVES
Obesity is a major risk factor for metabolic syndrome, a global public health problem. Mentha canadensis (MA), a traditional phytomedicine and dietary herb used for centuries, was the focus of this study to investigate its effects on obesity.
MATERIALS/METHODS
Thirty-five male C57BL/6J mice were randomly divided into 2 groups and fed either a normal diet (ND, n = 10) or a high-fat diet (HFD, n = 25) for 4 weeks to induce obesity. After the obesity induction period, the HFD-fed mice were randomly separated into 2 groups: one group continued to be fed HFD (n = 15, HFD group), while the other group was fed HFD with 1.5% (w/w) MA ethanol extract (n = 10, MA group) for 13 weeks.
RESULTS
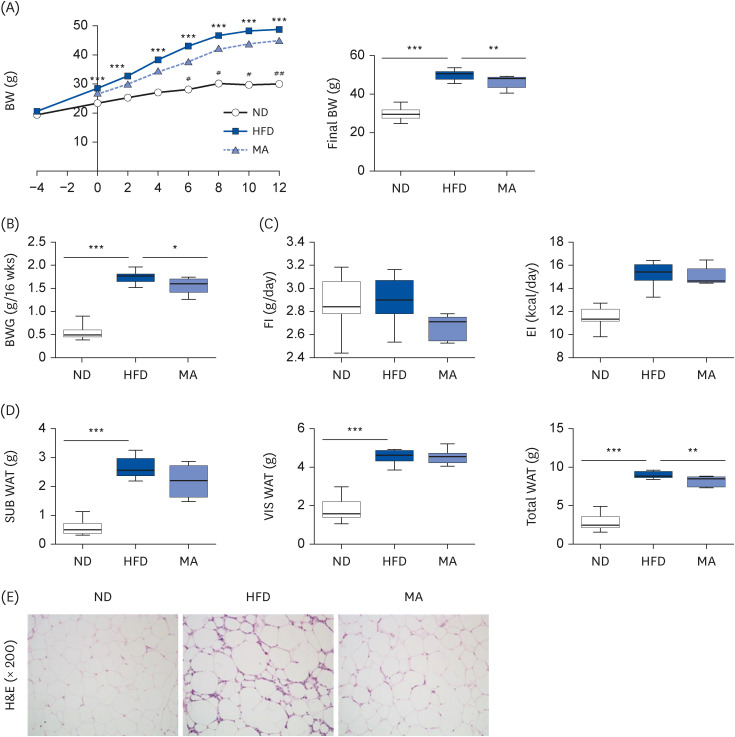
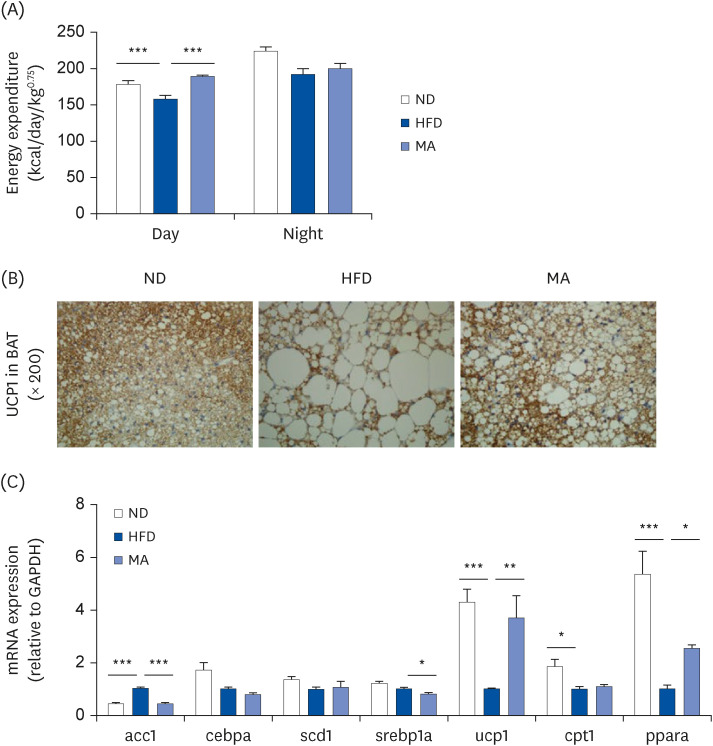
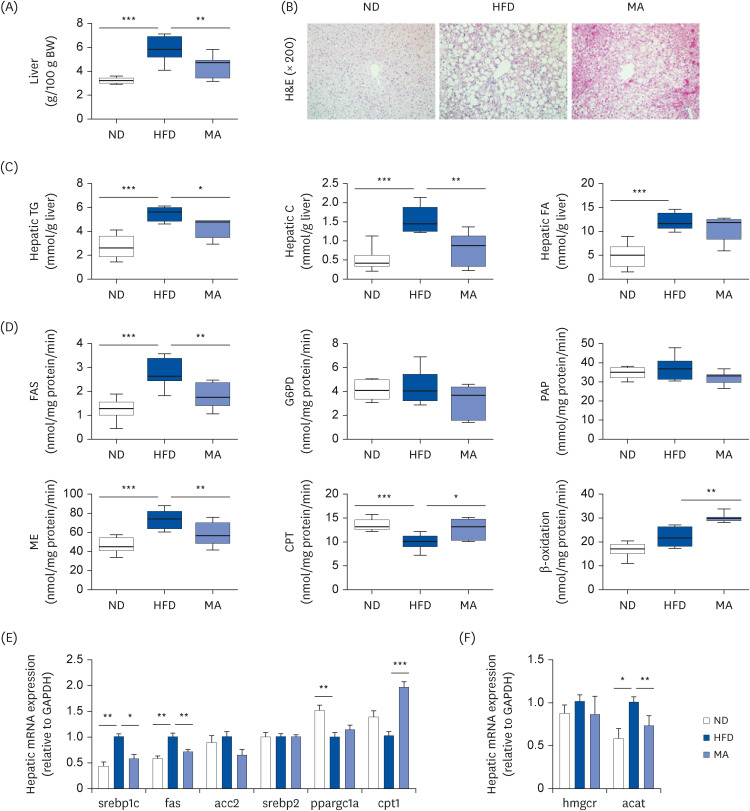
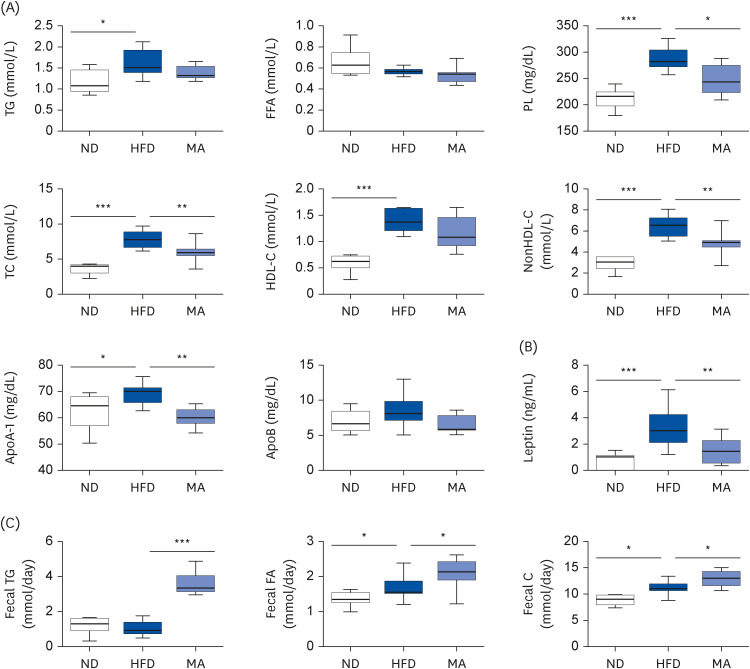
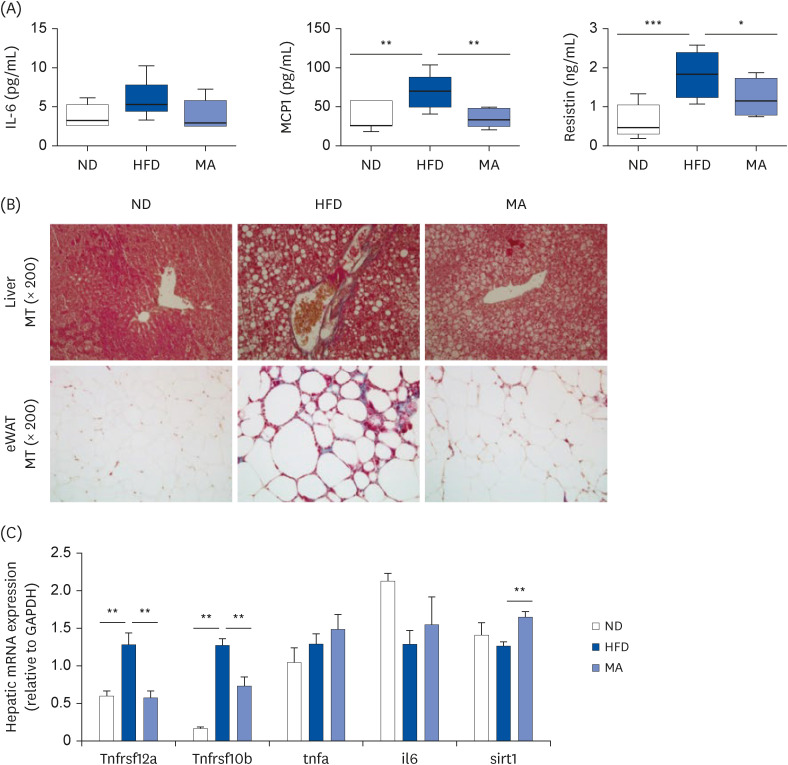
The results showed that body and white adipose tissue (WAT) weights were significantly decreased in the MA-supplemented group compared to the HFD group. Additionally, MA supplementation enhanced energy expenditure, leading to improvements in plasma lipids, cytokines, hepatic steatosis, and fecal lipids. Furthermore, MA supplementation regulated lipid-metabolism-related enzyme activity and gene expression, thereby suppressing lipid accumulation in the WAT and liver.
CONCLUSIONS
These findings indicate that MA has the potential to improve diet-induced obesity and its associated complications, including adiposity, dyslipidemia, hepatic steatosis, and inflammation.
Keyword
Figure
Reference
-
1. Bhurosy T, Jeewon R. Overweight and obesity epidemic in developing countries: a problem with diet, physical activity, or socioeconomic status? ScientificWorldJournal. 2014; 2014:964236. PMID: 25379554.2. Jung UJ, Choi MS. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci. 2014; 15:6184–6223. PMID: 24733068.3. Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology. 2010; 51:679–689. PMID: 20041406.4. Hasani-Ranjbar S, Nayebi N, Larijani B, Abdollahi M. A systematic review of the efficacy and safety of herbal medicines used in the treatment of obesity. World J Gastroenterol. 2009; 15:3073–3085. PMID: 19575486.5. Tucker AO, Chambers HL. Mentha canadensis L. (Lamiaceae): a relict amphidiploid from the Lower Tertiary. Taxon. 2002; 51:703–718.6. Hornok L. Cultivation and Processing of Medicinal Plants. Hoboken (NJ): Wiley;1992.7. Guenther E. The essential oils. New York (NY): D. Van Nostrand Company Inc.;1961.8. Barbalho SM, Spada AP, de Oliveira EP, Paiva-Filho ME, Martuchi KA, Leite NC, Deus RM, Sasaki V, Braganti LS, Oshiiwa M. Mentha piperita effects on Wistar rats plasma lipids. Braz Arch Biol Technol. 2009; 52:1137–1143.9. Yu X, Liang C, Chen J, Qi X, Liu Y, Li W. The effects of salinity stress on morphological characteristics, mineral nutrient accumulation and essential oil yield and composition in Mentha canadensis L. Sci Hortic (Amsterdam). 2015; 197:579–583.10. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957; 226:497–509. PMID: 13428781.11. Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007; 131:242–256. PMID: 17956727.12. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976; 72:248–254. PMID: 942051.13. Nepokroeff CM, Lakshmanan M, Porter JW. [6] Fatty acid synthase from rat liver. Methods Enzymol. 1975; 35:37–44. PMID: 1121291.14. Ochoa S, Mehler AH, Kornberg A. Biosynthesis of dicarboxylic acids by carbon dioxide fixation; isolation and properties of an enzyme from pigeon liver catalyzing the reversible oxidative decarboxylation of 1-malic acid. J Biol Chem. 1948; 174:979–1000. PMID: 18871257.15. Russo SB, Ross JS, Cowart LA. Sphingolipids in obesity, type 2 diabetes, and metabolic disease. Handb Exp Pharmacol. 2013; 373–401. PMID: 23563667.16. Pitkänen E, Pitkänen O, Uotila L. Enzymatic determination of unbound D-mannose in serum. Eur J Clin Chem Clin Biochem. 1997; 35:761–766. PMID: 9368794.17. Markwell MA, McGroarty EJ, Bieber LL, Tolbert NE. The subcellular distribution of carnitine acyltransferases in mammalian liver and kidney. A new peroxisomal enzyme. J Biol Chem. 1973; 248:3426–3432. PMID: 4702872.18. Walton PA, Possmayer F. The role of Mg2+-dependent phosphatidate phosphohydrolase in pulmonary glycerolipid biosynthesis. Biochim Biophys Acta. 1984; 796:364–372. PMID: 6095917.19. Han Y, Shin YC, Kim AH, Kwon EY, Choi MS. Evaluation of the dose-dependent effects of fermented mixed grain enzyme food on adiposity and its metabolic disorders in high-fat diet-induced obese mice. J Med Food. 2021; 24:873–882. PMID: 34406876.20. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001; 25:402–408. PMID: 11846609.21. Choi MS, Kim YJ, Kwon EY, Ryoo JY, Kim SR, Jung UJ. High-fat diet decreases energy expenditure and expression of genes controlling lipid metabolism, mitochondrial function and skeletal system development in the adipose tissue, along with increased expression of extracellular matrix remodelling- and inflammation-related genes. Br J Nutr. 2015; 113:867–877. PMID: 25744306.22. Hill JO, Wyatt HR, Peters JC. Energy balance and obesity. Circulation. 2012; 126:126–132. PMID: 22753534.23. Ikeda K, Yamada T. UCP1 dependent and independent thermogenesis in brown and beige adipocytes. Front Endocrinol (Lausanne). 2020; 11:498. PMID: 32849287.24. Roesler A, Kazak L. UCP1-independent thermogenesis. Biochem J. 2020; 477:709–725. PMID: 32059055.25. Ono-Moore KD, Rutkowsky JM, Pearson NA, Williams DK, Grobe JL, Tolentino T, Lloyd KC, Adams SH. Coupling of energy intake and energy expenditure across a temperature spectrum: impact of diet-induced obesity in mice. Am J Physiol Endocrinol Metab. 2020; 319:E472–E484. PMID: 32691631.26. Softic S, Cohen DE, Kahn CR. Role of dietary fructose and hepatic de novo lipogenesis in fatty liver disease. Dig Dis Sci. 2016; 61:1282–1293. PMID: 26856717.27. Boden G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes. 1997; 46:3–10. PMID: 8971073.28. Yki-Järvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014; 2:901–910. PMID: 24731669.29. Kwon EY, Jung UJ, Park T, Yun JW, Choi MS. Luteolin attenuates hepatic steatosis and insulin resistance through the interplay between the liver and adipose tissue in mice with diet-induced obesity. Diabetes. 2015; 64:1658–1669. PMID: 25524918.30. Lauressergues E, Martin F, Helleboid A, Bouchaert E, Cussac D, Bordet R, Hum D, Luc G, Majd Z, Staels B, et al. Overweight induced by chronic risperidone exposure is correlated with overexpression of the SREBP-1c and FAS genes in mouse liver. Naunyn Schmiedebergs Arch Pharmacol. 2011; 383:423–436. PMID: 21336545.31. Wakil SJ, Abu-Elheiga LA. Fatty acid metabolism: target for metabolic syndrome. J Lipid Res. 2009; 50(Suppl):S138–S143. PMID: 19047759.32. Monteiro R, Azevedo I. Chronic inflammation in obesity and the metabolic syndrome. Mediators Inflamm. 2010; 2010:289645. PMID: 20706689.33. Piya MK, McTernan PG, Kumar S. Adipokine inflammation and insulin resistance: the role of glucose, lipids and endotoxin. J Endocrinol. 2013; 216:T1–15. PMID: 23160966.34. Otero M, Lago R, Lago F, Casanueva FF, Dieguez C, Gómez-Reino JJ, Gualillo O. Leptin, from fat to inflammation: old questions and new insights. FEBS Lett. 2005; 579:295–301. PMID: 15642335.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- DA-6034 ameliorates hepatic steatosis and inflammation in high fat diet-induced obese mice
- Umbelliferone Ameliorates Hepatic Steatosis and Lipid-Induced ER Stress in High-Fat Diet-Induced Obese Mice
- Effects of the Combination of Evogliptin and Leucine on Insulin Resistance and Hepatic Steatosis in High-Fat Diet-Fed Mice
- Kaempferol ameliorates metabolic syndrome by inhibiting inflammation and oxidative stress in high-fat diet-induced obese mice
- Effect of Korean pine nut oil on hepatic iron, copper, and zinc status and expression of genes and proteins related to iron absorption in dietinduced obese mice