Nutr Res Pract.
2024 Jun;18(3):325-344. 10.4162/nrp.2024.18.3.325.
Kaempferol ameliorates metabolic syndrome by inhibiting inflammation and oxidative stress in high-fat diet-induced obese mice
- Affiliations
-
- 1Department of Food Science and Nutrition, Kyungpook National University, Daegu 41566, Korea
- 2Center for Food and Nutritional Genomics Research, Kyungpook National University, Daegu 41566, Korea
- 3Center for Beautiful Aging, Kyungpook National University, Daegu 41566, Korea
- KMID: 2558507
- DOI: http://doi.org/10.4162/nrp.2024.18.3.325
Abstract
- BACKGROUND/OBJECTIVES
Kaempferol (Ka) is one of the most widely occurring flavonoids found in large amounts in various plants. Ka has anti-obesity, antioxidant, and antiinflammatory effects. Despite the numerous papers documenting the efficacy of Ka, some controversy remains. Therefore, this study examined the impact of Ka using 3T3-L1 and highfat diet-induced obese mice.
MATERIALS/METHODS
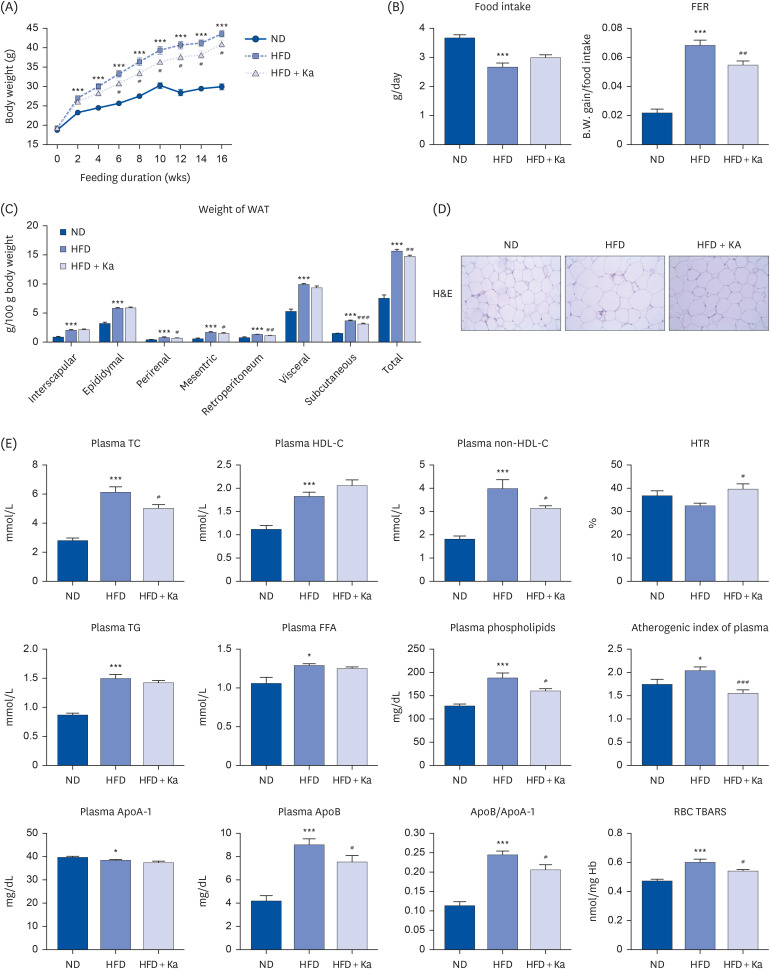
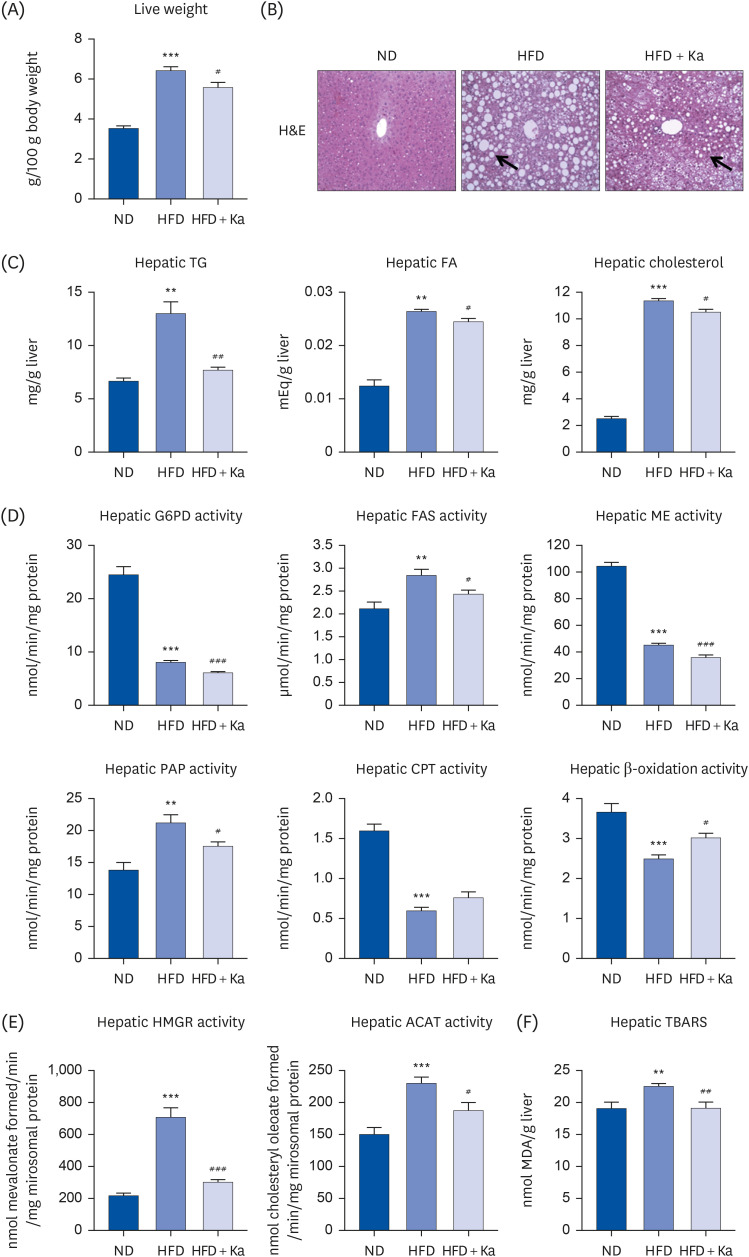
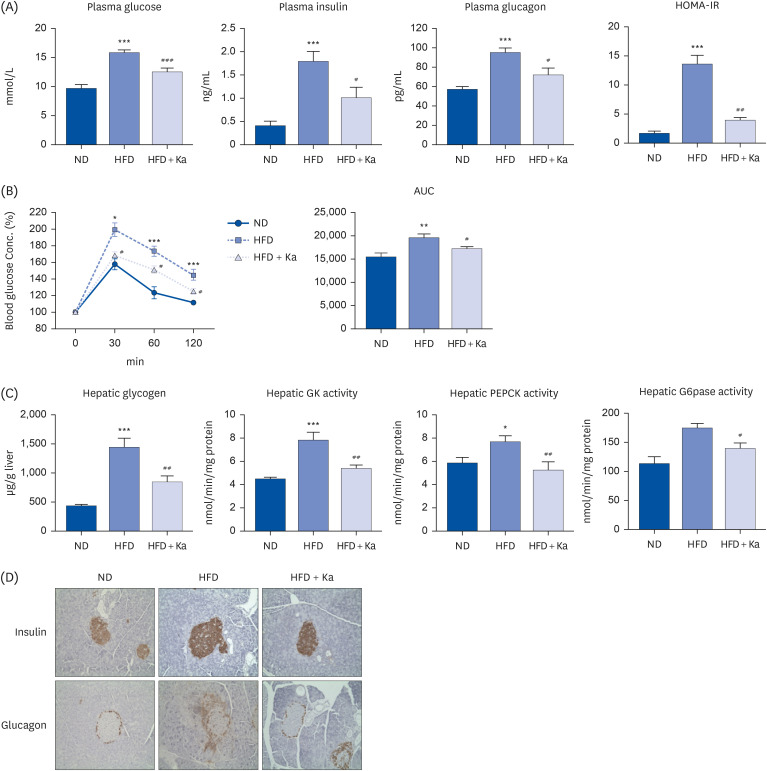
3T3-L1 cells were treated with 50 μM Ka from the initiation of 3T3-L1 differentiation at D0 until the completion of differentiation on D8. Thirty male mice (C57BL/6J, 4 weeks old) were divided into 3 groups: normal diet (ND), high-fat diet (HFD), and HFD + 0.02% (w/w) Ka (Ka) group. All mice were fed their respective diets ad libitum for 16 weeks. The mice were sacrificed, and the plasma and hepatic lipid levels, white adipose tissue weight, hepatic glucose level, lipid level, and antioxidant enzyme activities were analyzed, and immunohistochemistry staining was performed.
RESULTS
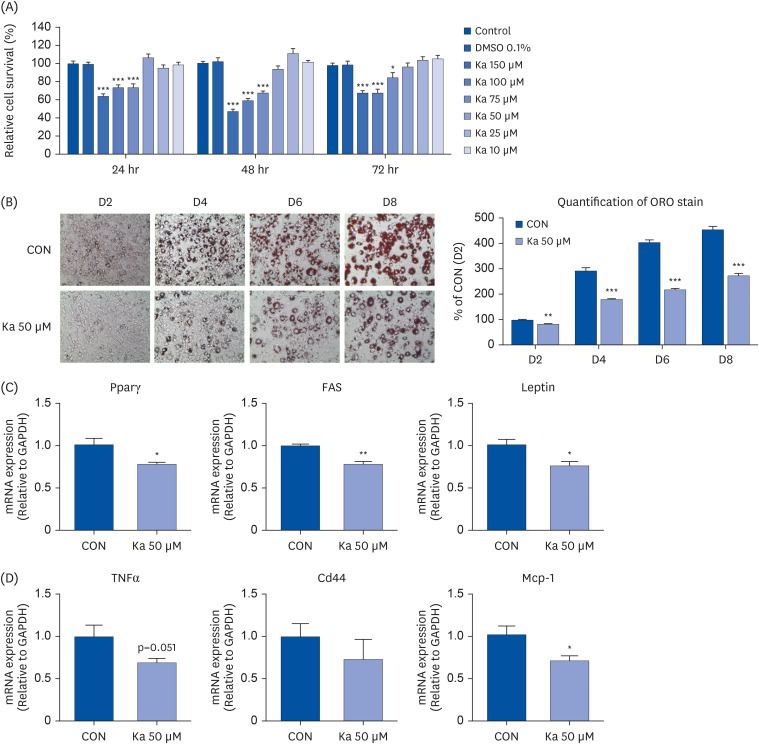
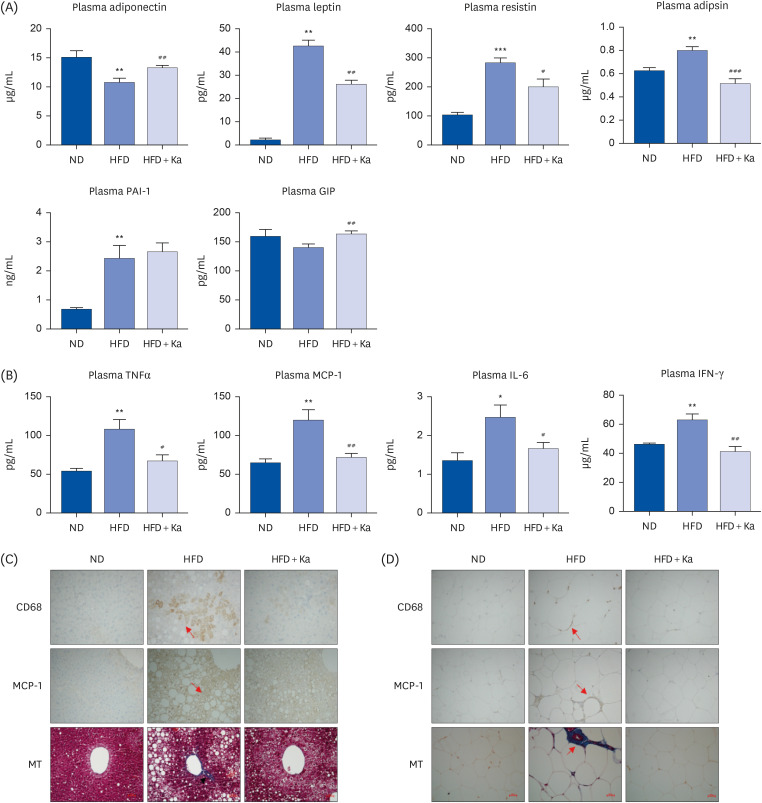
Ka suppressed the hypertrophy of 3T3-L1 cells, and the Ka-supplemented mice showed a significant decrease in perirenal, retroperitoneal, mesenteric, and subcutaneous fat compared to the HFD group. Ka supplementation in high-fat diet-induced obese mice also improved the overall blood lipid concentration (total cholesterol, non-high-density lipoprotein-cholesterol, phospholipids, and apolipoprotein B). Ka supplementation in highfat-induced obesity mice reduced hepatic steatosis and insulin resistance by modulating the hepatic lipid (glucose-6-phosphate dehydrogenase, fatty acid synthase, malic enzyme, phosphatidate phosphohydrolase, and β-oxidation) activities and glucose (glucokinase, phosphoenolpyruvate carboxykinase, and G6pase)-regulating enzymes. Ka supplementation ameliorated the erythrocyte and hepatic mitochondrial H2O2 and inflammation levels (plasma tumor necrosis factor-alpha, monocyte chemoattractant protein-1, interleukin-6, and interferon-gamma and fibrosis of liver and epididymal fat).
CONCLUSION
Ka may be beneficial for preventing diet-induced obesity, inflammation, oxidative stress, and diabetes.
Keyword
Figure
Reference
-
1. Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014; 384:766–781. PMID: 24880830.2. Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998; 83:847–850. PMID: 9506738.3. Shimomura I, Funahashi T, Takahashi M, Maeda K, Kotani K, Nakamura T, Yamashita S, Miura M, Fukuda Y, Takemura K, et al. Enhanced expression of PAI-1 in visceral fat: possible contributor to vascular disease in obesity. Nat Med. 1996; 2:800–803. PMID: 8673927.4. Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. The hormone resistin links obesity to diabetes. Nature. 2001; 409:307–312. PMID: 11201732.5. Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000; 106:473–481. PMID: 10953022.6. Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007; 132:2169–2180. PMID: 17498510.7. Huang Cao ZF, Stoffel E, Cohen P. Role of perivascular adipose tissue in vascular physiology and pathology. Hypertension. 2017; 69:770–777. PMID: 28320849.8. Reho JJ, Rahmouni K. Oxidative and inflammatory signals in obesity-associated vascular abnormalities. Clin Sci (Lond). 2017; 131:1689–1700. PMID: 28667067.9. Shah S, Iqbal M, Karam J, Salifu M, McFarlane SI. Oxidative stress, glucose metabolism, and the prevention of type 2 diabetes: pathophysiological insights. Antioxid Redox Signal. 2007; 9:911–929. PMID: 17508914.10. Park K, Gross M, Lee DH, Holvoet P, Himes JH, Shikany JM, Jacobs DR Jr. Oxidative stress and insulin resistance: the coronary artery risk development in young adults study. Diabetes Care. 2009; 32:1302–1307. PMID: 19389821.11. Jakubiak GK, Osadnik K, Lejawa M, Osadnik T, Goławski M, Lewandowski P, Pawlas N. “Obesity and insulin resistance” is the component of the metabolic syndrome most strongly associated with oxidative stress. Antioxidants (Basel). 2021; 11:79. PMID: 35052583.12. An G, Gallegos J, Morris ME. The bioflavonoid kaempferol is an Abcg2 substrate and inhibits Abcg2-mediated quercetin efflux. Drug Metab Dispos. 2011; 39:426–432. PMID: 21139040.13. Häkkinen SH, Kärenlampi SO, Heinonen IM, Mykkänen HM, Törrönen AR. Content of the flavonols quercetin, myricetin, and kaempferol in 25 edible berries. J Agric Food Chem. 1999; 47:2274–2279. PMID: 10794622.14. da-Silva WS, Harney JW, Kim BW, Li J, Bianco SD, Crescenzi A, Christoffolete MA, Huang SA, Bianco AC. The small polyphenolic molecule kaempferol increases cellular energy expenditure and thyroid hormone activation. Diabetes. 2007; 56:767–776. PMID: 17327447.15. Yu SF, Shun CT, Chen TM, Chen YH. 3-O-β-D-glucosyl-(1-->6)-β-D-glucosyl-kaempferol isolated from Sauropus androgenus reduces body weight gain in Wistar rats. Biol Pharm Bull. 2006; 29:2510–2513. PMID: 17142992.16. Lee YJ, Suh KS, Choi MC, Chon S, Oh S, Woo JT, Kim SW, Kim JW, Kim YS. Kaempferol protects HIT-T15 pancreatic beta cells from 2-deoxy-D-ribose-induced oxidative damage. Phytother Res. 2010; 24:419–423. PMID: 19827031.17. Suh KS, Choi EM, Kwon M, Chon S, Oh S, Woo JT, Kim SW, Kim JW, Kim YS. Kaempferol attenuates 2-deoxy-D-ribose-induced oxidative cell damage in MC3T3-E1 osteoblastic cells. Biol Pharm Bull. 2009; 32:746–749. PMID: 19336918.18. Crespo I, García-Mediavilla MV, Gutiérrez B, Sánchez-Campos S, Tuñón MJ, González-Gallego J. A comparison of the effects of kaempferol and quercetin on cytokine-induced pro-inflammatory status of cultured human endothelial cells. Br J Nutr. 2008; 100:968–976. PMID: 18394220.19. Hämäläinen M, Nieminen R, Vuorela P, Heinonen M, Moilanen E. Anti-inflammatory effects of flavonoids: genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-κB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-κB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediators Inflamm. 2007; 2007:45673. PMID: 18274639.20. Ninomiya K, Matsuda H, Kubo M, Morikawa T, Nishida N, Yoshikawa M. Potent anti-obese principle from Rosa canina: structural requirements and mode of action of trans-tiliroside. Bioorg Med Chem Lett. 2007; 17:3059–3064. PMID: 17400451.21. Christensen KB, Petersen RK, Kristiansen K, Christensen LP. Identification of bioactive compounds from flowers of black elder (Sambucus nigra L.) that activate the human peroxisome proliferator-activated receptor (PPAR) γ. Phytother Res. 2010; 24(Suppl 2):S129–S132. PMID: 20222152.22. Carroll NV, Longley RW, Roe JH. The determination of glycogen in liver and muscle by use of anthrone reagent. J Biol Chem. 1956; 220:583–593. PMID: 13331917.23. Davidson AL, Arion WJ. Factors underlying significant underestimations of glucokinase activity in crude liver extracts: physiological implications of higher cellular activity. Arch Biochem Biophys. 1987; 253:156–167. PMID: 3813560.24. Bentle LA, Lardy HA. Interaction of anions and divalent metal ions with phosphoenolpyruvate carboxykinase. J Biol Chem. 1976; 251:2916–2921. PMID: 1270433.25. Alegre M, Ciudad CJ, Fillat C, Guinovart JJ. Determination of glucose-6-phosphatase activity using the glucose dehydrogenase-coupled reaction. Anal Biochem. 1988; 173:185–189. PMID: 2847588.26. Nepokroeff CM, Lakshmanan MR, Porter JW. Fatty-acid synthase from rat liver. Methods Enzymol. 1975; 35:37–44. PMID: 1121291.27. Pitkänen E, Pitkänen O, Uotila L. Enzymatic determination of unbound D-mannose in serum. Eur J Clin Chem Clin Biochem. 1997; 35:761–766. PMID: 9368794.28. Markwell MA, McGroarty EJ, Bieber LL, Tolbert NE. The subcellular distribution of carnitine acyltransferases in mammalian liver and kidney. A new peroxisomal enzyme. J Biol Chem. 1973; 248:3426–3432. PMID: 4702872.29. Lazarow PB. Assay of peroxisomal beta-oxidation of fatty acids. Methods Enzymol. 1981; 72:315–319. PMID: 7031421.30. Shapiro DJ, Nordstrom JL, Mitschelen JJ, Rodwell VW, Schimke RT. Micro assay for 3-hydroxy-3-methylglutaryl-CoA reductase in rat liver and in L-cell fibroblasts. Biochim Biophys Acta. 1974; 370:369–377. PMID: 4441486.31. Gillies PJ, Rathgeb KA, Perri MA, Robinson CS. Regulation of acyl-CoA:cholesterol acyltransferase activity in normal and atherosclerotic rabbit aortas: role of a cholesterol substrate pool. Exp Mol Pathol. 1986; 44:329–339. PMID: 3720921.32. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979; 95:351–358. PMID: 36810.33. Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974; 47:469–474. PMID: 4215654.34. Aebi H. Catalase in vitro . Methods Enzymol. 1984; 105:121–126. PMID: 6727660.35. Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967; 70:158–169. PMID: 6066618.36. Torres-Villarreal D, Camacho A, Castro H, Ortiz-Lopez R, de la Garza AL. Anti-obesity effects of kaempferol by inhibiting adipogenesis and increasing lipolysis in 3T3-L1 cells. J Physiol Biochem. 2019; 75:83–88. PMID: 30539499.37. Aguilera CM, Gil-Campos M, Cañete R, Gil A. Alterations in plasma and tissue lipids associated with obesity and metabolic syndrome. Clin Sci (Lond). 2008; 114:183–193. PMID: 18184112.38. Pradhan A. Obesity, metabolic syndrome, and type 2 diabetes: inflammatory basis of glucose metabolic disorders. Nutr Rev. 2007; 65:S152–S156. PMID: 18240540.39. Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest. 2017; 127:1–4. PMID: 28045402.40. Bian Y, Lei J, Zhong J, Wang B, Wan Y, Li J, Liao C, He Y, Liu Z, Ito K, et al. Kaempferol reduces obesity, prevents intestinal inflammation, and modulates gut microbiota in high-fat diet mice. J Nutr Biochem. 2022; 99:108840. PMID: 34419569.41. Nainu F, Frediansyah A, Mamada SS, Permana AD, Salampe M, Chandran D, Emran TB, Simal-Gandara J. Natural products targeting inflammation-related metabolic disorders: a comprehensive review. Heliyon. 2023; 9:e16919. PMID: 37346355.42. Picchi A, Gao X, Belmadani S, Potter BJ, Focardi M, Chilian WM, Zhang C. Tumor necrosis factor-alpha induces endothelial dysfunction in the prediabetic metabolic syndrome. Circ Res. 2006; 99:69–77. PMID: 16741160.43. García-Ruiz I, Rodríguez-Juan C, Díaz-Sanjuan T, del Hoyo P, Colina F, Muñoz-Yagüe T, Solís-Herruzo JA. Uric acid and anti-TNF antibody improve mitochondrial dysfunction in ob/ob mice. Hepatology. 2006; 44:581–591. PMID: 16941682.44. McCullough AJ. Pathophysiology of nonalcoholic steatohepatitis. J Clin Gastroenterol. 2006; 40(Suppl 1):S17–S29. PMID: 16540762.45. Spranger J, Kroke A, Möhlig M, Hoffmann K, Bergmann MM, Ristow M, Boeing H, Pfeiffer AF. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes. 2003; 52:812–817. PMID: 12606524.46. Bastard JP, Maachi M, Van Nhieu JT, Jardel C, Bruckert E, Grimaldi A, Robert JJ, Capeau J, Hainque B. Adipose tissue IL-6 content correlates with resistance to insulin activation of glucose uptake both in vivo and in vitro . J Clin Endocrinol Metab. 2002; 87:2084–2089. PMID: 11994345.47. Juhan-Vague I, Alessi MC, Mavri A, Morange PE. Plasminogen activator inhibitor-1, inflammation, obesity, insulin resistance and vascular risk. J Thromb Haemost. 2003; 1:1575–1579. PMID: 12871293.48. Tarrats N, Moles A, Morales A, García-Ruiz C, Fernández-Checa JC, Marí M. Critical role of tumor necrosis factor receptor 1, but not 2, in hepatic stellate cell proliferation, extracellular matrix remodeling, and liver fibrogenesis. Hepatology. 2011; 54:319–327. PMID: 21523796.49. Martin S, Fernandez-Rojo MA, Stanley AC, Bastiani M, Okano S, Nixon SJ, Thomas G, Stow JL, Parton RG. Caveolin-1 deficiency leads to increased susceptibility to cell death and fibrosis in white adipose tissue: characterization of a lipodystrophic model. PLoS One. 2012; 7:e46242. PMID: 23049990.50. Arfian N, Setyaningsih WA, Romi MM, Sari DC. Heparanase upregulation from adipocyte associates with inflammation and endothelial injury in diabetic condition. BMC Proc. 2019; 13:17. PMID: 31890010.51. Chawla A, Nguyen KD, Goh YP. Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol. 2011; 11:738–749. PMID: 21984069.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inhibitory effects of Doenjang, Korean traditional fermented soybean paste, on oxidative stress and inflammation in adipose tissue of mice fed a high-fat diet
- Effects of caloric restriction on the expression of lipocalin-2 and its receptor in the brown adipose tissue of high-fat diet-fed mice
- Green perilla leaf extract ameliorates long-term oxidative stress induced by a high-fat diet in aging mice
- Effects of Voluntary Running-Wheel Exercise on Insulin, Oxidative Stress and Advanced Glycation End Products in High-Fat Diet-Induced Obese Mice
- The Inhibitory Effect of Quercetin on Adipose Tissue Inflammation in Mice Fed on a High-fat Diet