J Korean Neurosurg Soc.
2024 Mar;67(2):146-157. 10.3340/jkns.2023.0105.
Neovascularization in Outer Membrane of Chronic Subdural Hematoma : A Rationale for Middle Meningeal Artery Embolization
- Affiliations
-
- 1Department of Molecular Medicine and Biopharmaceutical Sciences, Graduate School of Convergence Science and Technology, College of Medicine or College of Pharmacy, Seoul National University, Seoul, Korea
- 2Department of Nuclear Medicine, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- 3Department of Pathology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- 4Department of Neurosurgery, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- 5Radiation Medicine Institute, Seoul National University College of Medicine, Seoul, Korea
- 6Institute on Aging, Seoul National University, Seoul, Korea
- KMID: 2553120
- DOI: http://doi.org/10.3340/jkns.2023.0105
Abstract
Objective
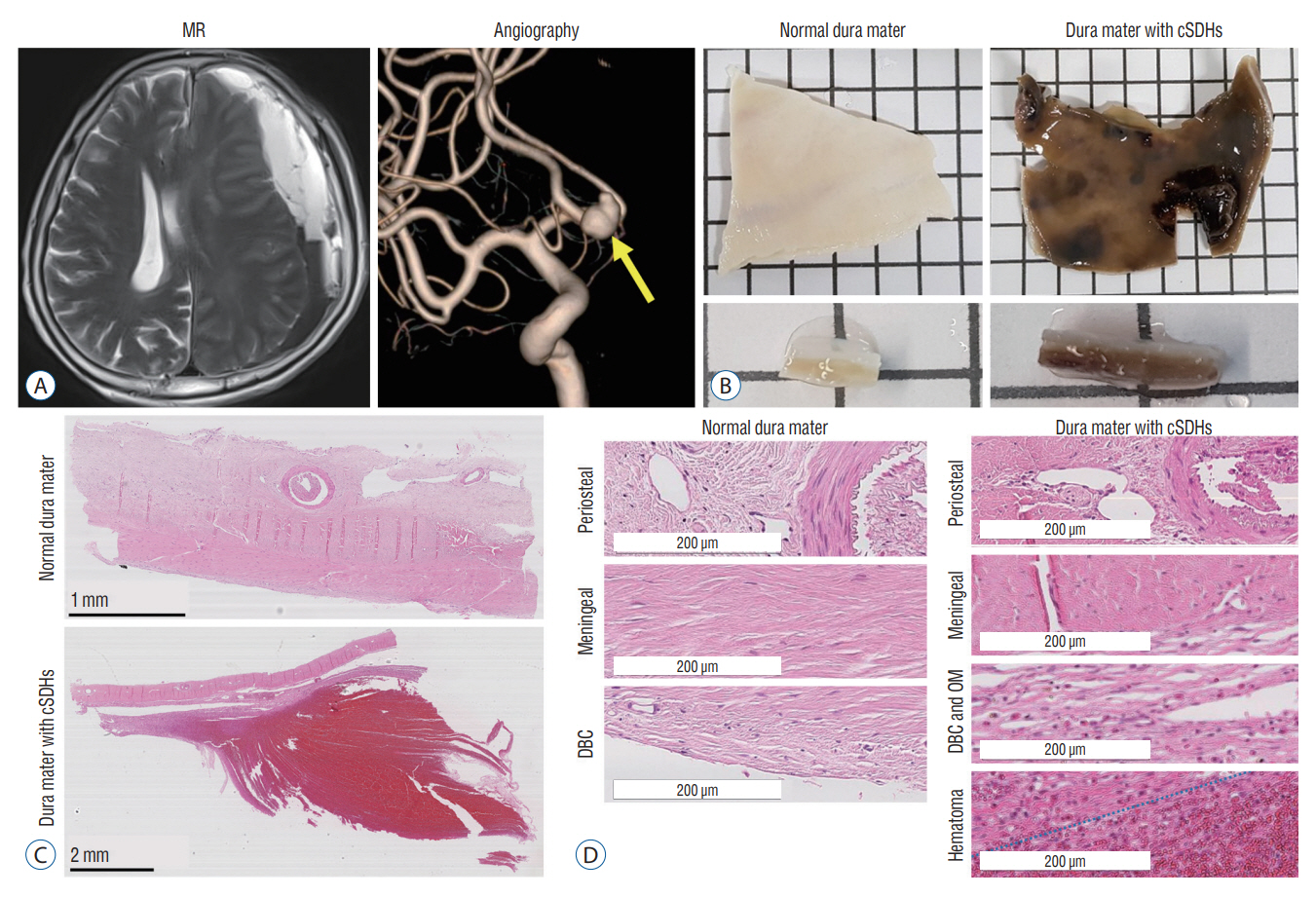
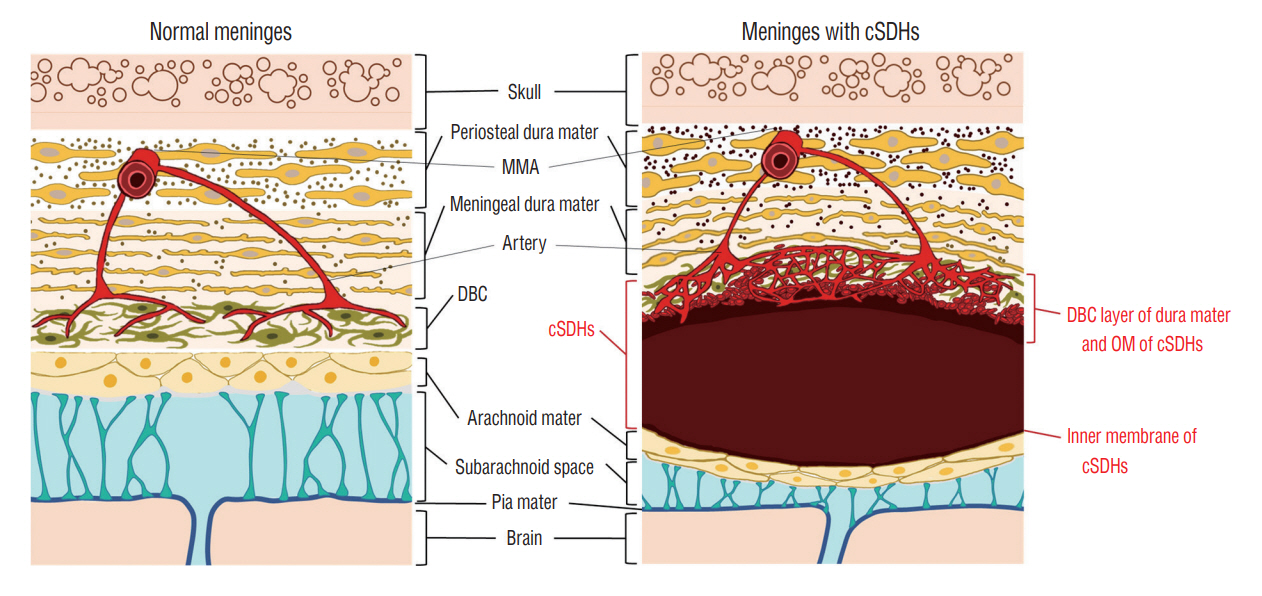
: Chronic subdural hematomas (cSDHs) are generally known to result from traumatic tears of bridging veins. However, the causes of repeat spontaneous cSDHs are still unclear. We investigated the changes in vasculature in the human dura mater and outer membrane (OM) of cSDHs to elucidate the cause of their spontaneous repetition.
Methods
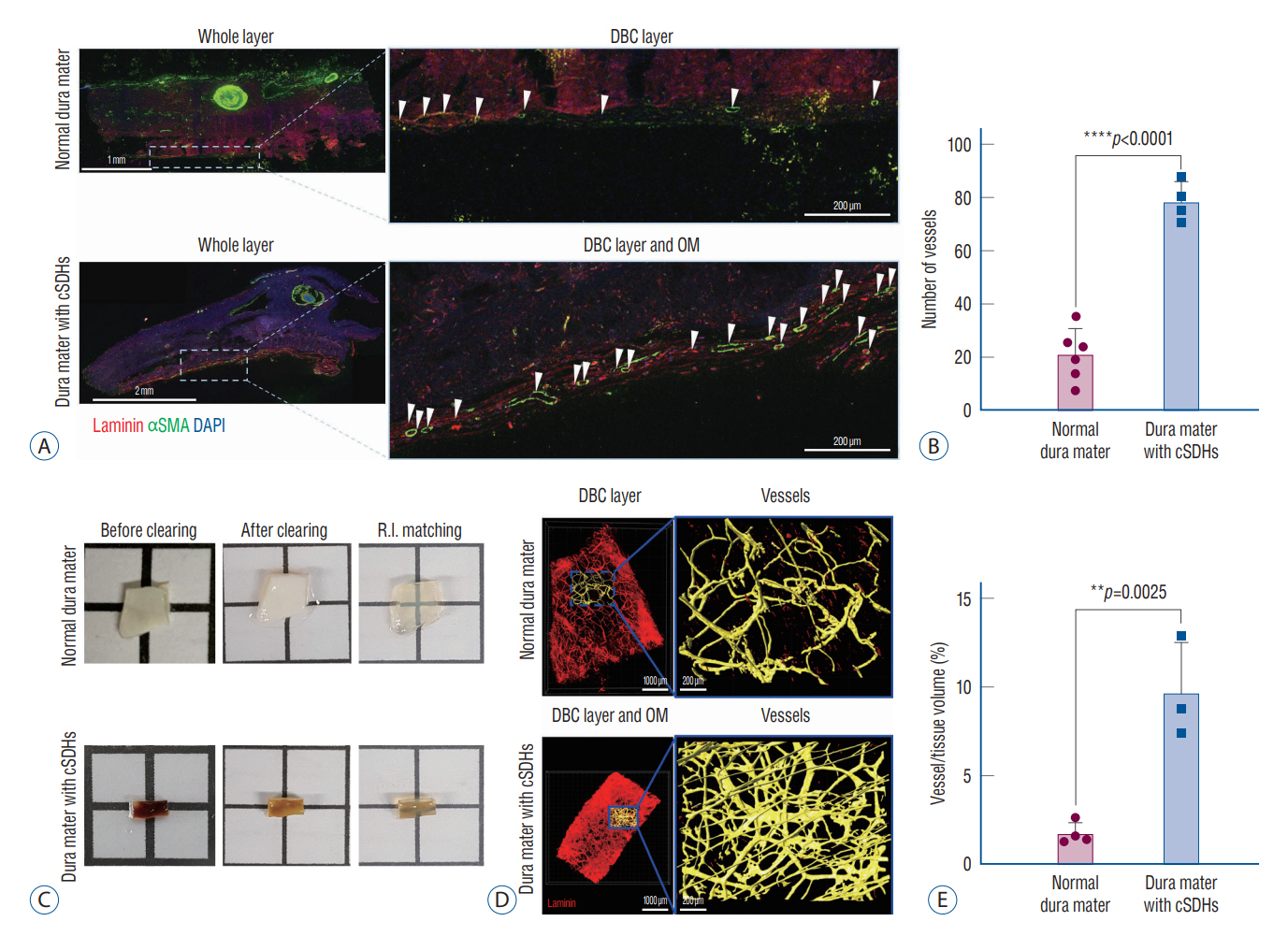
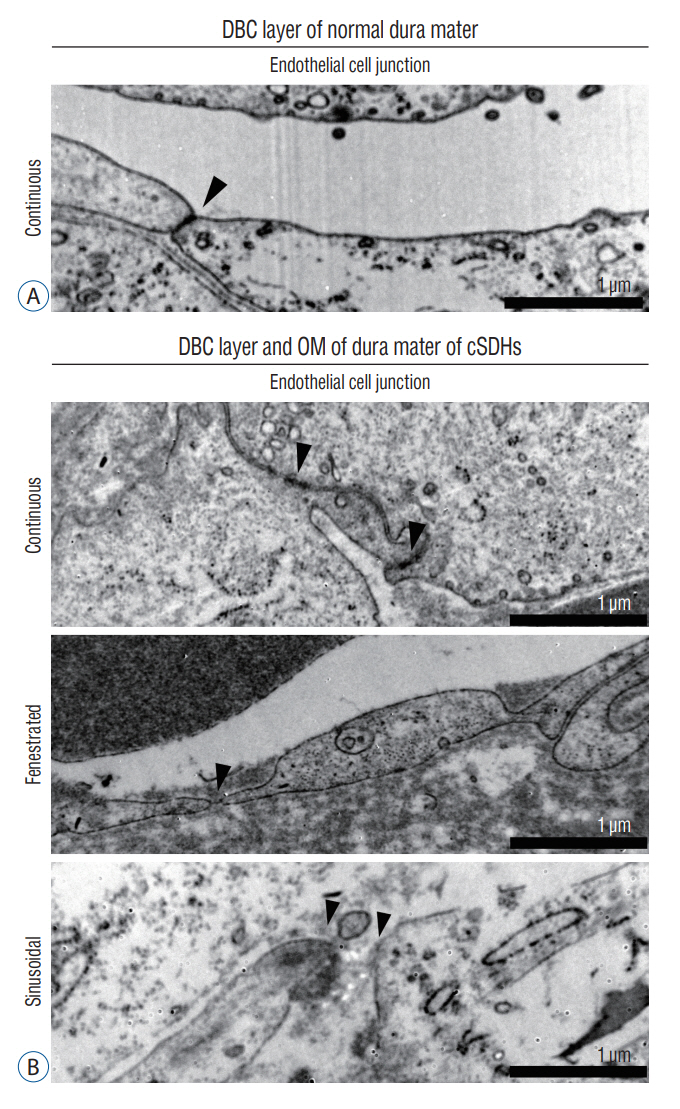
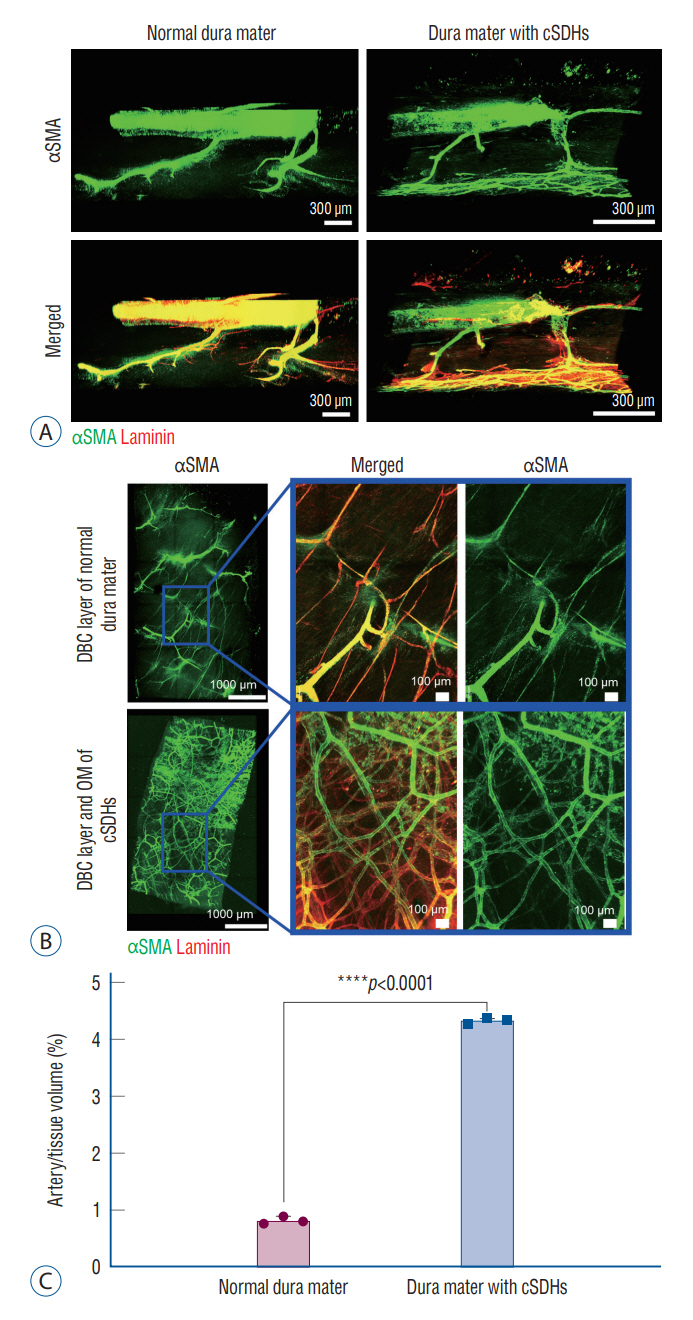
: The dura mater was obtained from a normal control participant and a patient with repeat spontaneous cSDHs. The pathological samples from the patient included the dura mater and OM tightly adhered to the inner dura. The samples were analyzed with a particular focus on blood and lymphatic vessels by immunohistochemistry, 3-dimensional imaging using a transparent tissue clearing technique, and electron microscopy.
Results
: The dural border cell (DBC) layer of the dura mater and OM were histologically indistinguishable. There were 5.9 times more blood vessels per unit volume of tissue in the DBC layer and OM in the patient than in the normal control. The DBC layer and OM contained pathological sinusoidal capillaries not observed in the normal tissue; these capillaries were connected to the middle meningeal arteries via penetrating arteries. In addition, marked lymphangiogenesis in the periosteal and meningeal layers was observed in the patient with cSDHs.
Conclusion
: Neovascularization in the OM seemed to originate from the DBC layer; this is a potential cause of repeat spontaneous cSDHs. Embolization of the meningeal arteries to interrupt the blood supply to pathological capillaries via penetrating arteries may be an effective treatment option.
Keyword
Figure
Reference
-
References
1. Augustin HG, Koh GY. Organotypic vasculature: from descriptive heterogeneity to functional pathophysiology. Science. 357:eaal2379. 2017.2. Ban SP, Hwang G, Byoun HS, Kim T, Lee SU, Bang JS, et al. Middle meningeal artery embolization for chronic subdural hematoma. Radiology. 286:992–999. 2018.3. Blanco R, Gerhardt H. VEGF and Notch in tip and stalk cell selection. Cold Spring Harb Perspect Med. 3:a006569. 2013.4. Bolte AC, Dutta AB, Hurt ME, Smirnov I, Kovacs MA, McKee CA, et al. Meningeal lymphatic dysfunction exacerbates traumatic brain injury pathogenesis. Nat Commun. 11:4524. 2020.5. Chen JC, Levy ML. Causes, epidemiology, and risk factors of chronic subdural hematoma. Neurosurg Clin N Am. 11:399–406. 2000.6. Cho WS, Batchuluun B, Lee SJ, Kang HS, Kim JE. Recurrent subdural hematoma from a pseudoaneurysm at the cortical branch of the middle cerebral artery after mild head injury: case report. Neurol Med Chir (Tokyo). 51:217–221. 2011.7. Edlmann E, Giorgi-Coll S, Whitfield PC, Carpenter KLH, Hutchinson PJ. Pathophysiology of chronic subdural haematoma: inflammation, angiogenesis and implications for pharmacotherapy. J Neuroinflammation. 14:108. 2017.8. Fiorella D, Arthur AS. Middle meningeal artery embolization for the management of chronic subdural hematoma. J Neurointerv Surg. 11:912–915. 2019.9. Gardner WJ. Traumatic subdural hematoma: with particular reference to the latent interval. AMA Arch Neurol Psychiatry. 27:847–858. 1932.10. Haldrup M, Ketharanathan B, Debrabant B, Schwartz OS, Mikkelsen R, Fugleholm K, et al. Embolization of the middle meningeal artery in patients with chronic subdural hematoma-a systematic review and metaanalysis. Acta Neurochir (Wien). 162:777–784. 2020.11. Hirashima Y, Kurimoto M, Nagai S, Hori E, Origasa H, Endo S. Effect of platelet-activating factor receptor antagonist, etizolam, on resolution of chronic subdural hematoma--a prospective study to investigate use as conservative therapy. Neurol Med Chir (Tokyo). 45:621–626. discussion 626. 2005.12. Ito H, Yamamoto S, Komai T, Mizukoshi H. Role of local hyperfibrinolysis in the etiology of chronic subdural hematoma. J Neurosurg. 45:26–31. 1976.13. Kageyama H, Toyooka T, Tsuzuki N, Oka K. Nonsurgical treatment of chronic subdural hematoma with tranexamic acid. J Neurosurg. 119:332–337. 2013.14. Kawakami Y, Chikama M, Tamiya T, Shimamura Y. Coagulation and fibrinolysis in chronic subdural hematoma. Neurosurgery. 25:25–29. 1989.15. Koh BI, Lee HJ, Kwak PA, Yang MJ, Kim JH, Kim HS, et al. VEGFR2 signaling drives meningeal vascular regeneration upon head injury. Nat Commun. 11:3866. 2020.16. Link TW, Boddu S, Paine SM, Kamel H, Knopman J. Middle meningeal artery embolization for chronic subdural hematoma: a series of 60 cases. Neurosurgery. 85:801–807. 2019.17. Liu X, Gao C, Yuan J, Xiang T, Gong Z, Luo H, et al. Subdural haematomas drain into the extracranial lymphatic system through the meningeal lymphatic vessels. Acta Neuropathol Commun. 8:16. 2020.18. Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 523:337–341. 2015.19. Mehta V, Harward SC, Sankey EW, Nayar G, Codd PJ. Evidence based diagnosis and management of chronic subdural hematoma: a review of the literature. J Clin Neurosci. 50:7–15. 2018.20. Ohba S, Kinoshita Y, Nakagawa T, Murakami H. The risk factors for recurrence of chronic subdural hematoma. Neurosurg Rev. 36:145–149. discussion 149-150. 2013.21. Parlato C, Guarracino A, Moraci A. Spontaneous resolution of chronic subdural hematoma. Surg Neurol. 53:312–315. discussion 315-317. 2000.22. Rambaud C. Bridging veins and autopsy findings in abusive head trauma. Pediatr Radiol. 45:1126–1131. 2015.23. Rauhala M, Luoto TM, Huhtala H, Iverson GL, Niskakangas T, Öhman J, et al. The incidence of chronic subdural hematomas from 1990 to 2015 in a defined Finnish population. J Neurosurg. 132:1147–1157. 2019.24. Sahyouni R, Goshtasbi K, Mahmoodi A, Tran DK, Chen JW. Chronic subdural hematoma: a perspective on subdural membranes and dementia. World Neurosurg. 108:954–958. 2017.25. Shapiro M, Walker M, Carroll KT, Levitt MR, Raz E, Nossek E, et al. Neuroanatomy of cranial dural vessels: implications for subdural hematoma embolization. J Neurointerv Surg. 13:471–477. 2021.26. Shim YS, Park CO, Hyun DK, Park HC, Yoon SH. What are the causative factors for a slow, progressive enlargement of a chronic subdural hematoma? Yonsei Med J. 48:210–217. 2007.27. Sim YW, Min KS, Lee MS, Kim YG, Kim DH. Recent changes in risk factors of chronic subdural hematoma. J Korean Neurosurg Soc. 52:234–239. 2012.28. Srivatsan A, Mohanty A, Nascimento FA, Hafeez MU, Srinivasan VM, Thomas A, et al. Middle meningeal artery embolization for chronic subdural hematoma: meta-analysis and systematic review. World Neurosurg. 122:613–619. 2019.29. Stanisic M, Aasen AO, Pripp AH, Lindegaard KF, Ramm-Pettersen J, Lyngstadaas SP, et al. Local and systemic pro-inflammatory and antiinflammatory cytokine patterns in patients with chronic subdural hematoma: a prospective study. Inflamm Res. 61:845–852. 2012.30. Wang D, Gao C, Xu X, Chen T, Tian Y, Wei H, et al. Treatment of chronic subdural hematoma with atorvastatin combined with low-dose dexamethasone: phase II randomized proof-of-concept clinical trial. J Neurosurg. 134:235–243. 2020.31. Weigel R, Hohenstein A, Schlickum L, Weiss C, Schilling L. Angiotensin converting enzyme inhibition for arterial hypertension reduces the risk of recurrence in patients with chronic subdural hematoma possibly by an antiangiogenic mechanism. Neurosurgery. 61:788–792. discussion 792-793. 2007.32. Weigel R, Schilling L, Schmiedek P. Specific pattern of growth factor distribution in chronic subdural hematoma (CSH): evidence for an angiogenic disease. Acta Neurochir (Wien). 143:811–818. discussion 819. 2001.33. Yamashima T, Yamamoto S, Friede RL. The role of endothelial gap junctions in the enlargement of chronic subdural hematomas. J Neurosurg. 59:298–303. 1983.34. Yang W, Huang J. Chronic subdural hematoma: epidemiology and natural history. Neurosurg Clin N Am. 28:205–210. 2017.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Endovascular surgery for chronic subdural hematoma
- Usefulness of Middle Meningeal Embolization to Prevent Recurrent Spontaneous Chronic Subdural Hemorrhage
- Retrograde Middle Meningeal Artery Embolization through Mini Craniotomy for Subdural Hematoma Evacuation: A Technical Note
- Middle meningeal artery embolization for postoperative supratentorial chronic subdural hematoma occurring after posterior fossa neurosurgery
- Middle Meningeal Artery Embolization in Recurrent Chronic Subdural Hematoma Combined with Arachnoid Cyst