Endocrinol Metab.
2024 Feb;39(1):98-108. 10.3803/EnM.2023.1786.
Inhibition of Sodium-Glucose Cotransporter-2 during Serum Deprivation Increases Hepatic Gluconeogenesis via the AMPK/AKT/FOXO Signaling Pathway
- Affiliations
-
- 1Institute of Medical Research, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea
- 2Division of Endocrinology and Metabolism, Department of Internal Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2552802
- DOI: http://doi.org/10.3803/EnM.2023.1786
Abstract
- Background
Sodium-dependent glucose cotransporter 2 (SGLT2) mediates glucose reabsorption in the renal proximal tubules, and SGLT2 inhibitors are used as therapeutic agents for treating type 2 diabetes mellitus. This study aimed to elucidate the effects and mechanisms of SGLT2 inhibition on hepatic glucose metabolism in both serum deprivation and serum supplementation states.
Methods
Huh7 cells were treated with the SGLT2 inhibitors empagliflozin and dapagliflozin to examine the effect of SGLT2 on hepatic glucose uptake. To examine the modulation of glucose metabolism by SGLT2 inhibition under serum deprivation and serum supplementation conditions, HepG2 cells were transfected with SGLT2 small interfering RNA (siRNA), cultured in serum-free Dulbecco’s modified Eagle’s medium for 16 hours, and then cultured in media supplemented with or without 10% fetal bovine serum for 8 hours.
Results
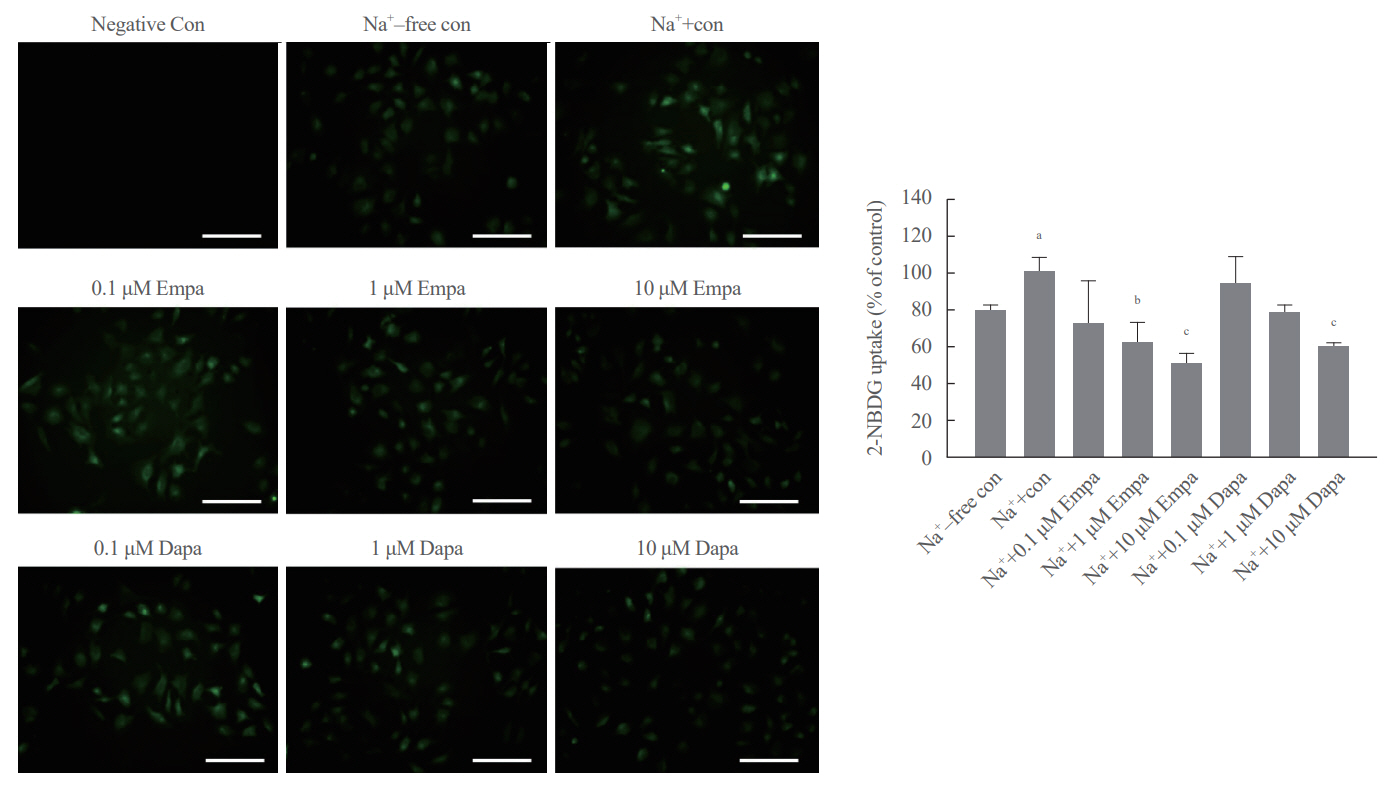
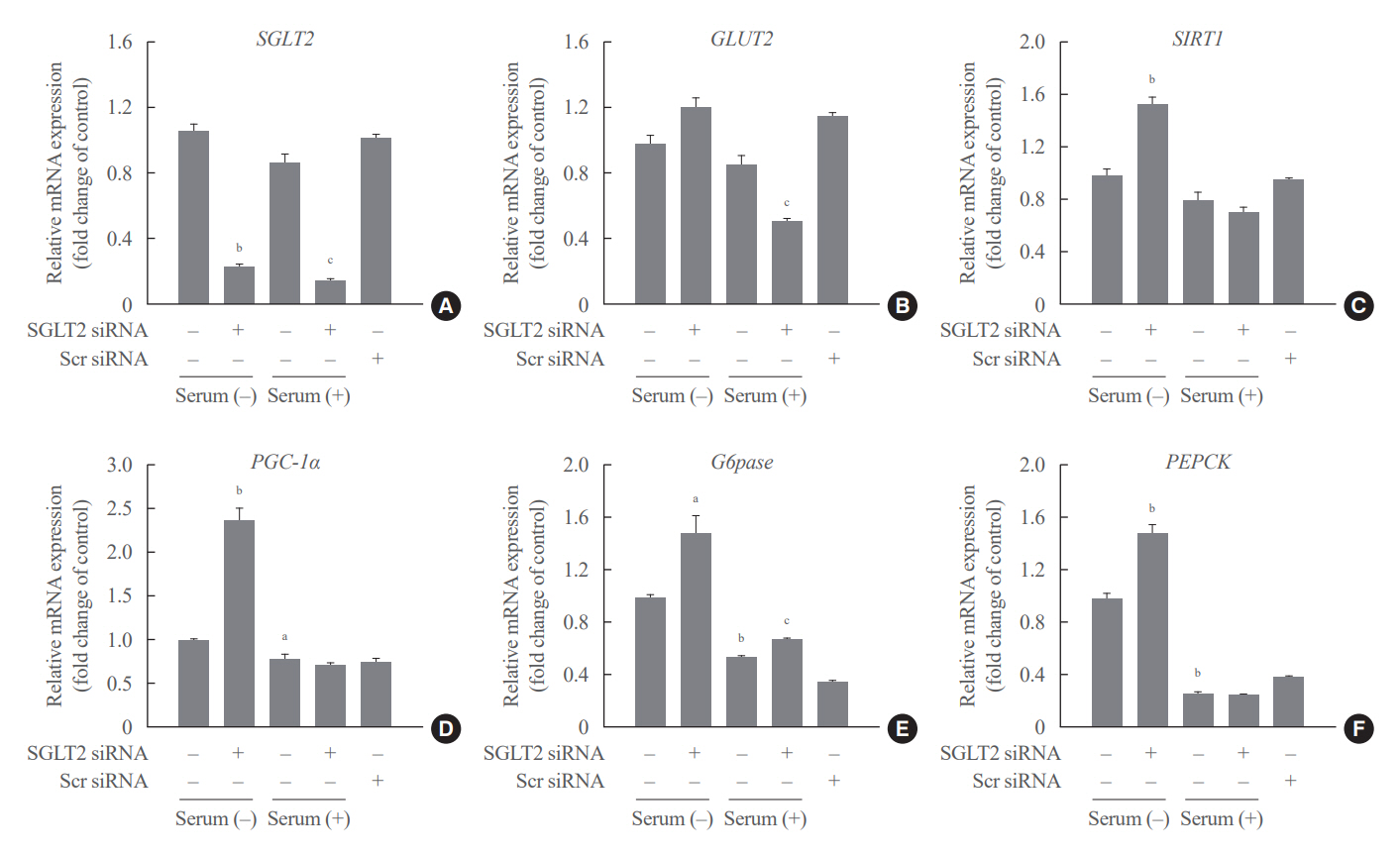
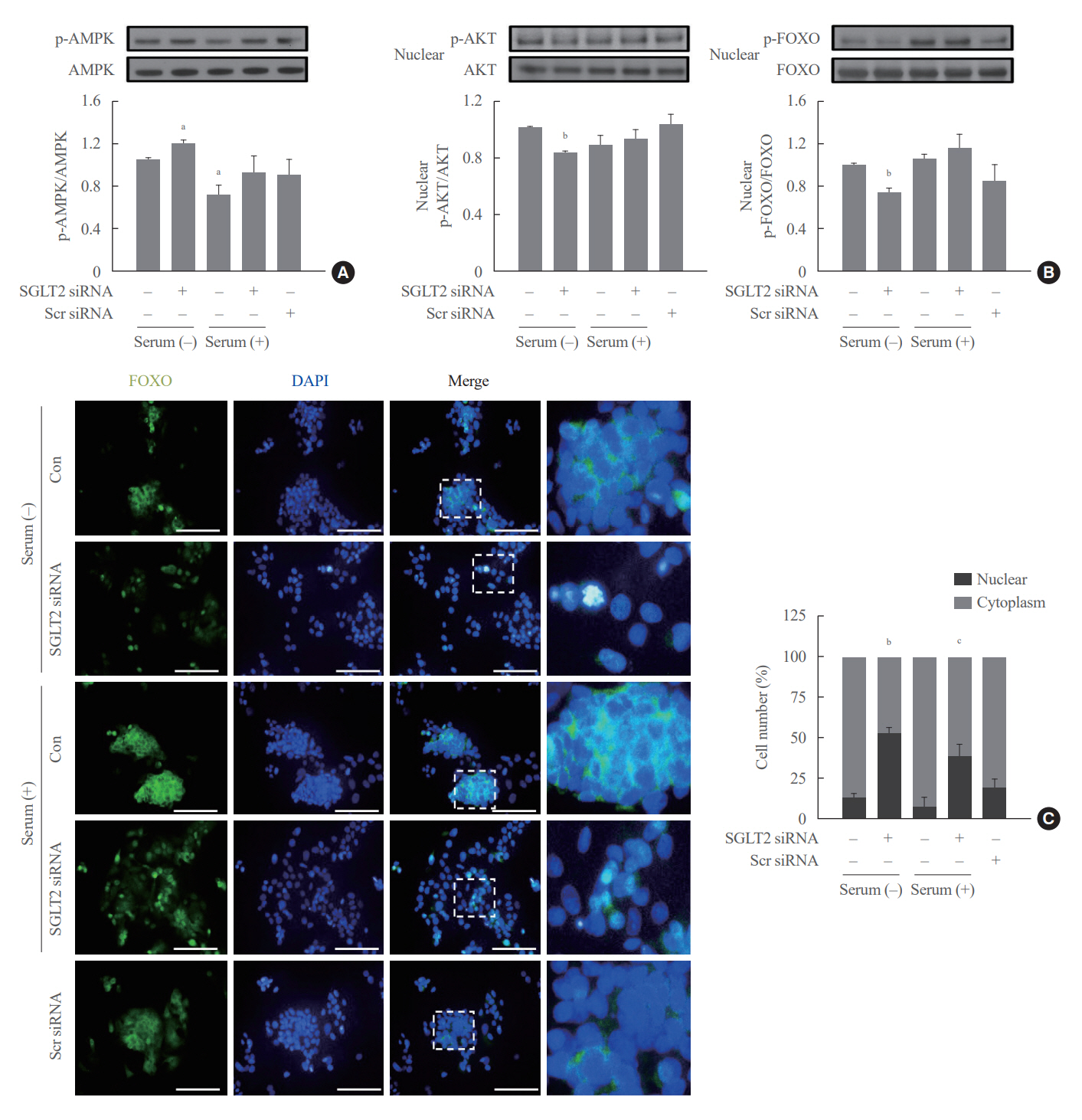
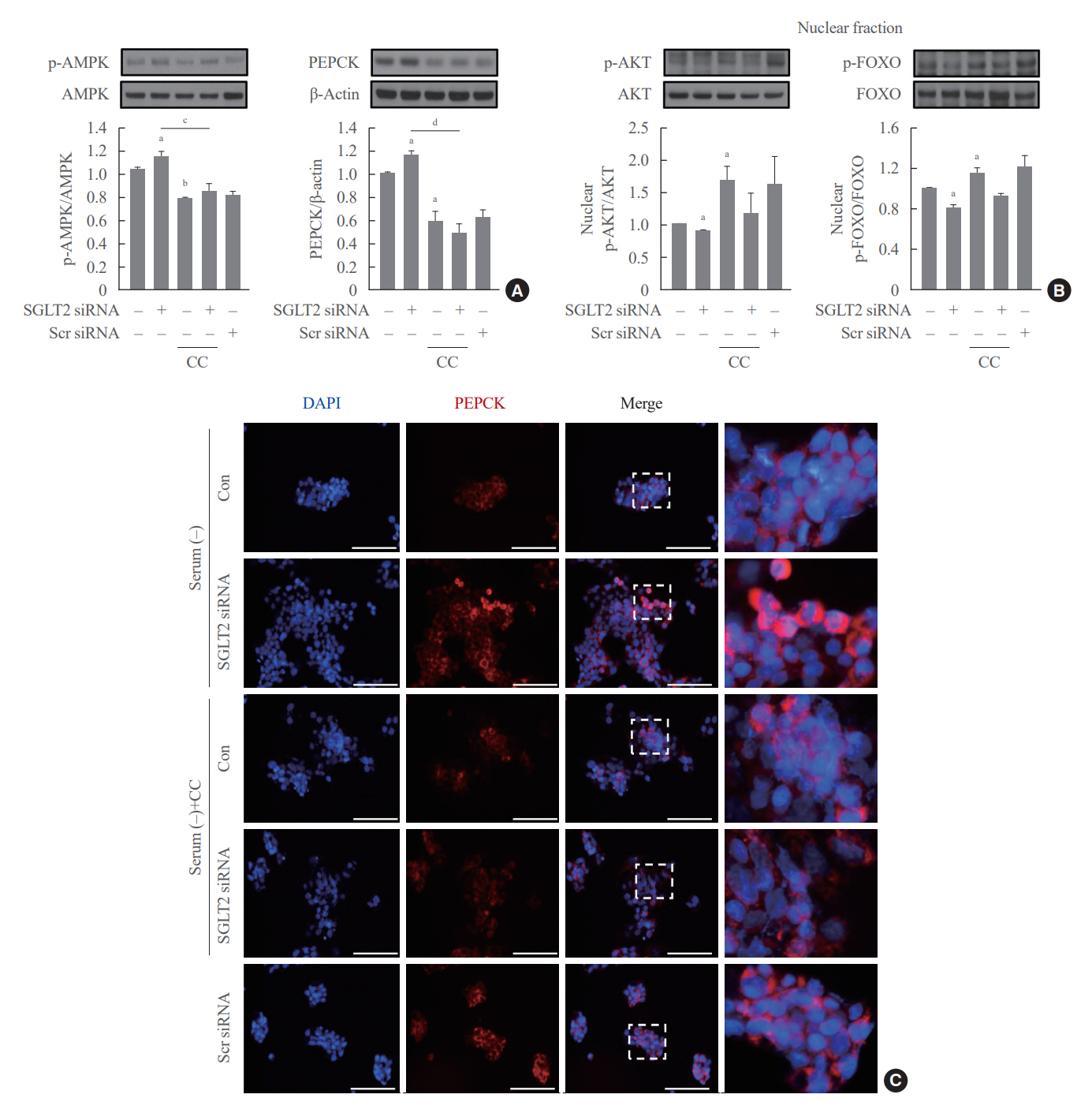
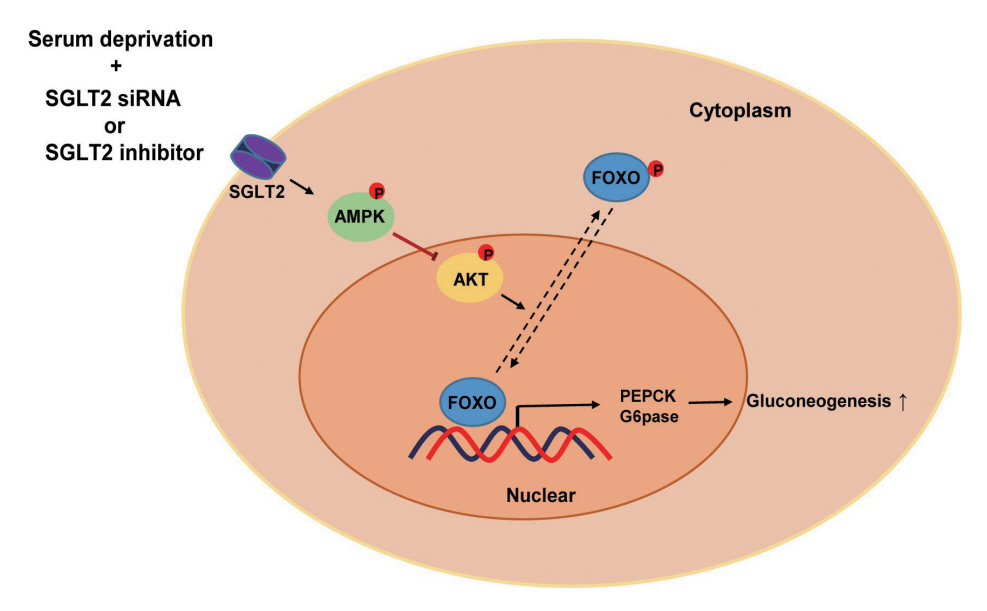
SGLT2 inhibitors dose-dependently decreased hepatic glucose uptake. Serum deprivation increased the expression levels of the gluconeogenesis genes peroxisome proliferator-activated receptor gamma co-activator 1 alpha (PGC-1α), glucose 6-phosphatase (G6pase), and phosphoenolpyruvate carboxykinase (PEPCK), and their expression levels during serum deprivation were further increased in cells transfected with SGLT2 siRNA. SGLT2 inhibition by siRNA during serum deprivation induces nuclear localization of the transcription factor forkhead box class O 1 (FOXO1), decreases nuclear phosphorylated-AKT (p-AKT), and p-FOXO1 protein expression, and increases phosphorylated-adenosine monophosphate-activated protein kinase (p-AMPK) protein expression. However, treatment with the AMPK inhibitor, compound C, reversed the reduction in the protein expression levels of nuclear p- AKT and p-FOXO1 and decreased the protein expression levels of p-AMPK and PEPCK in cells transfected with SGLT2 siRNA during serum deprivation.
Conclusion
These data show that SGLT2 mediates glucose uptake in hepatocytes and that SGLT2 inhibition during serum deprivation increases gluconeogenesis via the AMPK/AKT/FOXO1 signaling pathway.
Keyword
Figure
Reference
-
1. Adeva-Andany MM, Perez-Felpete N, Fernandez-Fernandez C, Donapetry-Garcia C, Pazos-Garcia C. Liver glucose metabolism in humans. Biosci Rep. 2016; 36:e00416.2. Sano R, Shinozaki Y, Ohta T. Sodium-glucose cotransporters: functional properties and pharmaceutical potential. J Diabetes Investig. 2020; 11:770–82.3. Vallon V, Platt KA, Cunard R, Schroth J, Whaley J, Thomson SC, et al. SGLT2 mediates glucose reabsorption in the early proximal tubule. J Am Soc Nephrol. 2011; 22:104–12.4. Kim JH, Ko HY, Wang HJ, Lee H, Yun M, Kang ES. Effect of dapagliflozin, a sodium-glucose co-transporter-2 inhibitor, on gluconeogenesis in proximal renal tubules. Diabetes Obes Metab. 2020; 22:373–82.5. Obara K, Shirakami Y, Maruta A, Ideta T, Miyazaki T, Kochi T, et al. Preventive effects of the sodium glucose cotransporter 2 inhibitor tofogliflozin on diethylnitrosamine-induced liver tumorigenesis in obese and diabetic mice. Oncotarget. 2017; 8:58353–63.6. Li L, Li Q, Huang W, Han Y, Tan H, An M, et al. Dapagliflozin alleviates hepatic steatosis by restoring autophagy via the AMPK-mTOR pathway. Front Pharmacol. 2021; 12:589273.7. Akirov A, Grossman A, Shochat T, Shimon I. Mortality among hospitalized patients with hypoglycemia: insulin related and noninsulin related. J Clin Endocrinol Metab. 2017; 102:416–24.8. Nakhleh A, Shehadeh N. Hypoglycemia in diabetes: an update on pathophysiology, treatment, and prevention. World J Diabetes. 2021; 12:2036–49.9. Anderson M, Powell J, Campbell KM, Taylor JR. Optimal management of type 2 diabetes in patients with increased risk of hypoglycemia. Diabetes Metab Syndr Obes. 2014; 7:85–94.10. Filippas-Ntekouan S, Filippatos TD, Elisaf MS. SGLT2 inhibitors: are they safe? Postgrad Med. 2018; 130:72–82.11. Merovci A, Solis-Herrera C, Daniele G, Eldor R, Fiorentino TV, Tripathy D, et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest. 2014; 124:509–14.12. Zhang X, Yang S, Chen J, Su Z. Unraveling the regulation of hepatic gluconeogenesis. Front Endocrinol (Lausanne). 2019; 9:802.13. Geisler CE, Hepler C, Higgins MR, Renquist BJ. Hepatic adaptations to maintain metabolic homeostasis in response to fasting and refeeding in mice. Nutr Metab (Lond). 2016; 13:62.14. Rui L. Energy metabolism in the liver. Compr Physiol. 2014; 4:177–97.15. Cano N. Bench-to-bedside review: glucose production from the kidney. Crit Care. 2002; 6:317–21.16. Gerich JE. Role of the kidney in normal glucose homeostasis and in the hyperglycaemia of diabetes mellitus: therapeutic implications. Diabet Med. 2010; 27:136–42.17. Furukawa-Hibi Y, Kobayashi Y, Chen C, Motoyama N. FOXO transcription factors in cell-cycle regulation and the response to oxidative stress. Antioxid Redox Signal. 2005; 7:752–60.18. Wang Y, Zhou Y, Graves DT. FOXO transcription factors: their clinical significance and regulation. Biomed Res Int. 2014; 2014:925350.19. Tikhanovich I, Cox J, Weinman SA. Forkhead box class O transcription factors in liver function and disease. J Gastroenterol Hepatol. 2013; 28 Suppl 1:125–31.20. Miyauchi T, Uchida Y, Kadono K, Hirao H, Kawasoe J, Watanabe T, et al. Up-regulation of FOXO1 and reduced inflammation by β-hydroxybutyric acid are essential diet restriction benefits against liver injury. Proc Natl Acad Sci U S A. 2019; 116:13533–42.21. Kousteni S. FoxO1, the transcriptional chief of staff of energy metabolism. Bone. 2012; 50:437–43.22. Lu YT, Ma XL, Xu YH, Hu J, Wang F, Qin WY, et al. A fluorescent glucose transport assay for screening SGLT2 inhibitors in endogenous SGLT2-expressing HK-2 cells. Nat Prod Bioprospect. 2019; 9:13–21.23. Zhang X, Tang N, Hadden TJ, Rishi AK. Akt, FoxO and regulation of apoptosis. Biochim Biophys Acta. 2011; 1813:1978–86.24. Tzivion G, Dobson M, Ramakrishnan G. FoxO transcription factors; regulation by AKT and 14-3-3 proteins. Biochim Biophys Acta. 2011; 1813:1938–45.25. Zhou Y, Yu H, Cheng S, Chen Y, He L, Ren J, et al. Glutamate dehydrogenase 1 mediated glutaminolysis sustains HCC cells survival under glucose deprivation. J Cancer. 2022; 13:1061–72.26. Oh KJ, Han HS, Kim MJ, Koo SH. CREB and FoxO1: two transcription factors for the regulation of hepatic gluconeogenesis. BMB Rep. 2013; 46:567–74.27. Barthel A, Schmoll D. Novel concepts in insulin regulation of hepatic gluconeogenesis. Am J Physiol Endocrinol Metab. 2003; 285:E685–92.28. Moon JS, Won KC. Pancreatic α-cell dysfunction in type 2 diabetes: old kids on the block. Diabetes Metab J. 2015; 39:1–9.29. Bonner C, Kerr-Conte J, Gmyr V, Queniat G, Moerman E, Thevenet J, et al. Inhibition of the glucose transporter SGLT2 with dapagliflozin in pancreatic alpha cells triggers glucagon secretion. Nat Med. 2015; 21:512–7.30. Yang Y, Chen S, Pan H, Zou Y, Wang B, Wang G, et al. Safety and efficiency of SGLT2 inhibitor combining with insulin in subjects with diabetes: systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2017; 96:e6944.31. Link W, Fernandez-Marcos PJ. FOXO transcription factors at the interface of metabolism and cancer. Int J Cancer. 2017; 141:2379–91.32. Aoyama H, Daitoku H, Fukamizu A. Nutrient control of phosphorylation and translocation of Foxo1 in C57BL/6 and db/db mice. Int J Mol Med. 2006; 18:433–9.33. Puigserver P, Rhee J, Donovan J, Walkey CJ, Yoon JC, Oriente F, et al. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1alpha interaction. Nature. 2003; 423:550–5.34. He L, Li Y, Zeng N, Stiles BL. Regulation of basal expression of hepatic PEPCK and G6Pase by AKT2. Biochem J. 2020; 477:1021–31.35. Zhao Y, Hu X, Liu Y, Dong S, Wen Z, He W, et al. ROS signaling under metabolic stress: cross-talk between AMPK and AKT pathway. Mol Cancer. 2017; 16:79.36. Guo X, Li X, Yang W, Liao W, Shen JZ, Ai W, et al. Metformin targets Foxo1 to control glucose homeostasis. Biomolecules. 2021; 11:873.37. Zhao X, Gan L, Pan H, Kan D, Majeski M, Adam SA, et al. Multiple elements regulate nuclear/cytoplasmic shuttling of FOXO1: characterization of phosphorylation- and 14-3-3-dependent and -independent mechanisms. Biochem J. 2004; 378(Pt 3):839–49.38. Karim S, Adams DH, Lalor PF. Hepatic expression and cellular distribution of the glucose transporter family. World J Gastroenterol. 2012; 18:6771–81.39. Chadt A, Al-Hasani H. Glucose transporters in adipose tissue, liver, and skeletal muscle in metabolic health and disease. Pflugers Arch. 2020; 472:1273–98.40. Zhang W, Liu HT. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002; 12:9–18.41. Lu R, Wang X, Chen ZF, Sun DF, Tian XQ, Fang JY. Inhibition of the extracellular signal-regulated kinase/mitogen-activated protein kinase pathway decreases DNA methylation in colon cancer cells. J Biol Chem. 2007; 282:12249–59.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Repeated Glucose Deprivation/Reperfusion Induced PC-12 Cell Death through the Involvement of FOXO Transcription Factor
- The Role of the Kidney in Glucose Metabolism
- Anti-hyperglycemic effects and signaling mechanism of Perilla frutescens sprout extract
- AMPK Activator AICAR Inhibits Hepatic Gluconeogenesis and Fatty Acid Oxidation
- Glucose Lowering Effect of SGLT2 Inhibitors: A Review of Clinical Studies