Intest Res.
2024 Jan;22(1):65-74. 10.5217/ir.2023.00092.
Combination of leucine-rich alpha-2 glycoprotein and fecal markers detect Crohn’s disease activity confirmed by balloon-assisted enteroscopy
- Affiliations
-
- 1Department of Gastroenterology and Hepatology, Tokyo Medical and Dental University, Tokyo, Japan
- 2Department of Endoscopic Unit, Tokyo Medical and Dental University, Tokyo, Japan
- 3Department of Radiology, Tokyo Medical and Dental University, Tokyo, Japan
- KMID: 2551279
- DOI: http://doi.org/10.5217/ir.2023.00092
Abstract
- Background/Aims
Endoscopic activity confirmed by enteroscopy is associated with poor clinical outcome in Crohn’s disease (CD). We investigated which of the existing biomarkers best reflects endoscopic activity in CD patients including the small bowel, and whether their combined use can improve accuracy.
Methods
One hundred and four consecutive patients with ileal and ileocolonic type CD who underwent balloon-assisted enteroscopy (BAE) from October 2021 to August 2022 were enrolled, with clinical and laboratory data prospectively collected and analyzed.
Results
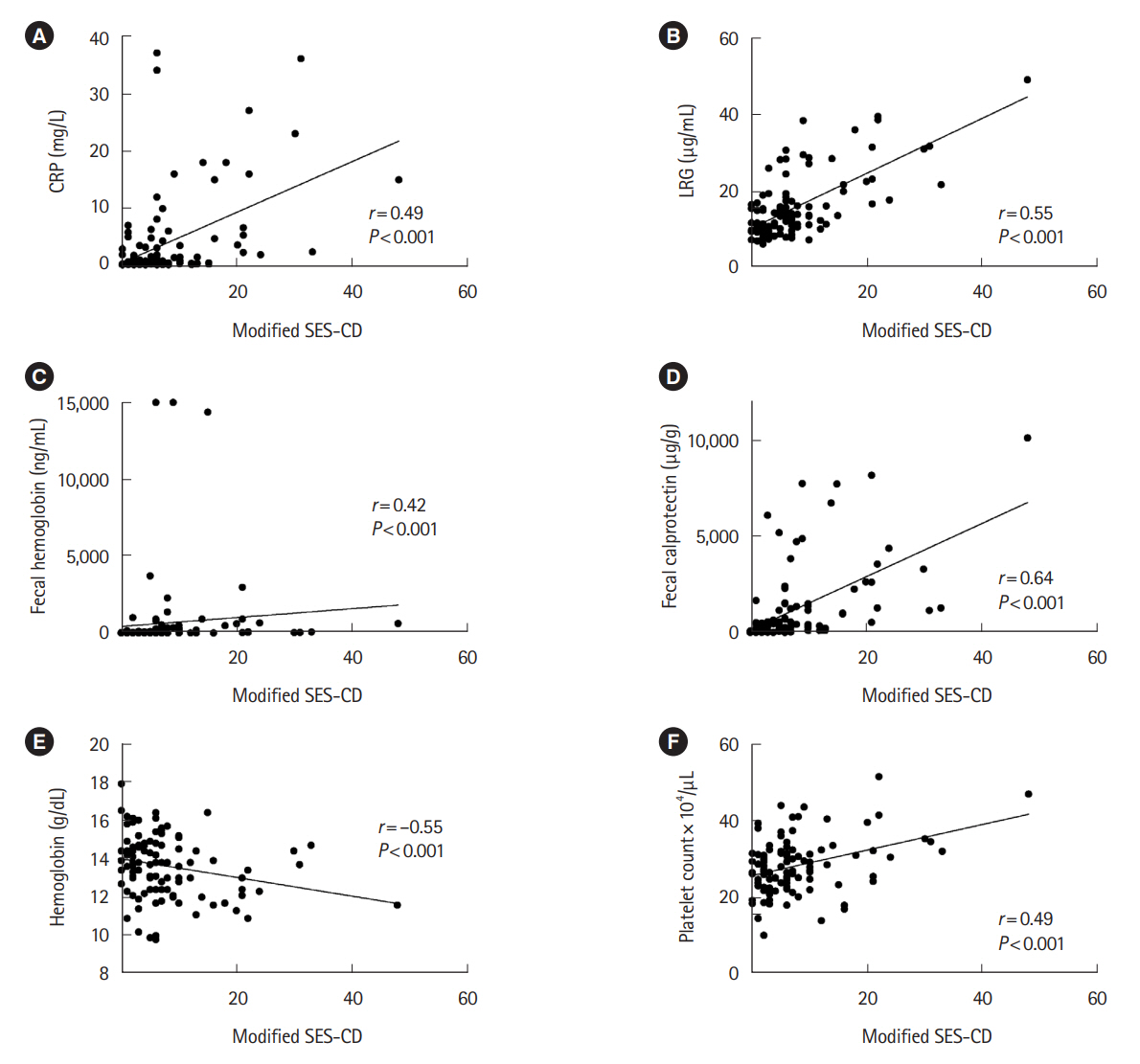
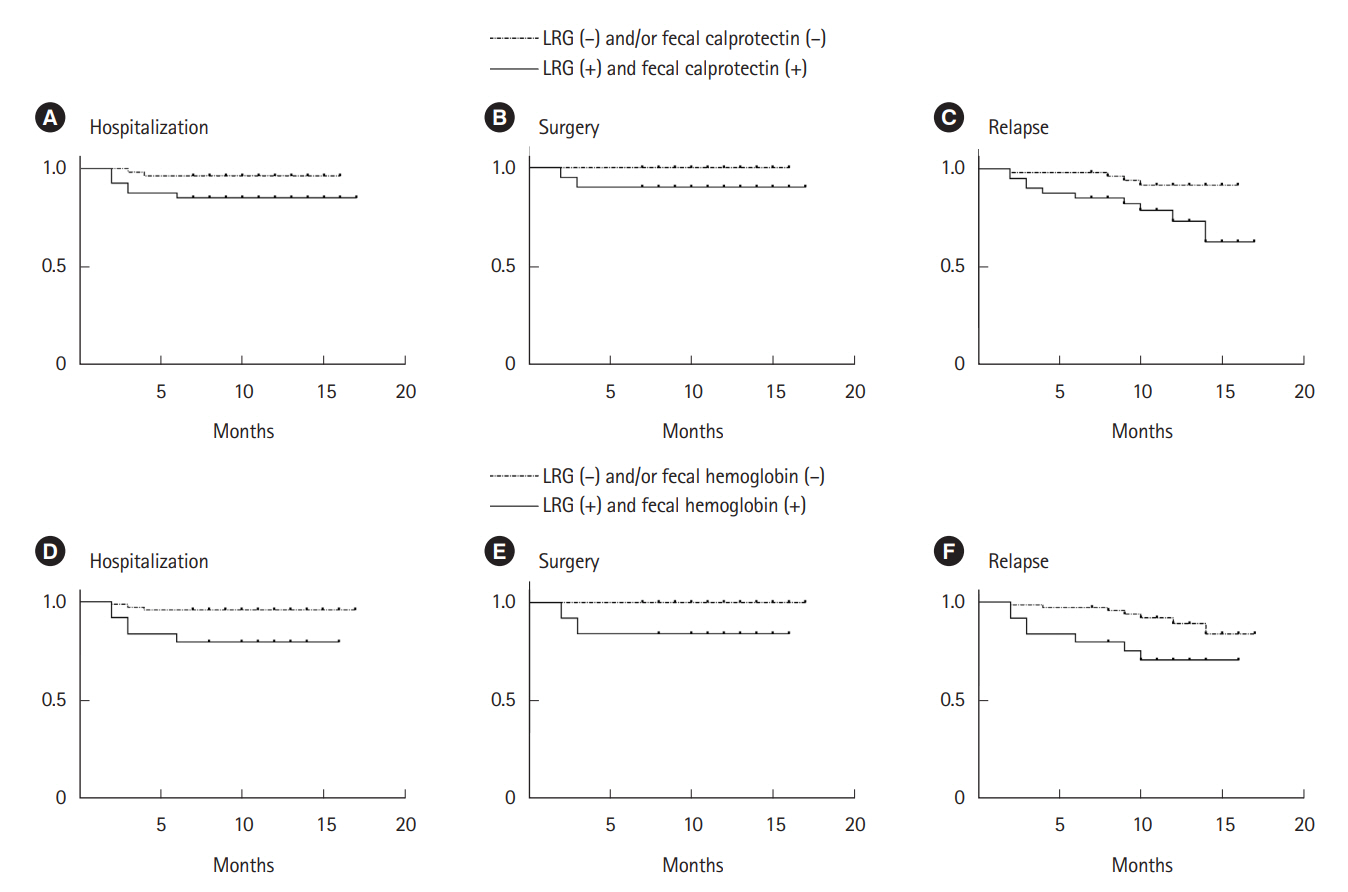
Hemoglobin, platelet count, C-reactive protein, leucine-rich alpha-2 glycoprotein (LRG), fecal calprotectin, and fecal hemoglobin all showed significant difference in those with ulcers found on BAE. LRG and fecal calprotectin showed the highest areas under the curve (0.841 and 0.853) for detecting ulcers. LRG showed a sensitivity of 78% and specificity of 80% at a cutoff value of 13 μg/mL, whereas fecal calprotectin showed a sensitivity of 91% and specificity of 67% at a cutoff value of 151 μg/g. Dual positivity for LRG and fecal calprotectin, as well as LRG and fecal hemoglobin, both predicted ulcers with an improved specificity of 92% and 100%. A positive LRG or fecal calprotectin/hemoglobin showed an improved sensitivity of 96% and 91%. Positivity for LRG and either of the fecal biomarkers was associated with increased risk of hospitalization, surgery, and relapse.
Conclusions
The biomarkers LRG, fecal calprotectin, and fecal hemoglobin can serve as noninvasive and accurate tools for assessing activity in CD patients confirmed by BAE, especially when used in combination.
Keyword
Figure
Reference
-
1. Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021; 160:1570–1583.
Article2. Takenaka K, Ohtsuka K, Kitazume Y, et al. Utility of magnetic resonance enterography for small bowel endoscopic healing in patients with Crohn’s disease. Am J Gastroenterol. 2018; 113:283–294.
Article3. Colombel JF, Panaccione R, Bossuyt P, et al. Effect of tight control management on Crohn’s disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet. 2017; 390:2779–2789.
Article4. Simon EG, Wardle R, Thi AA, Eldridge J, Samuel S, Moran GW. Does fecal calprotectin equally and accurately measure disease activity in small bowel and large bowel Crohn’s disease? A systematic review. Intest Res. 2019; 17:160–170.
Article5. Iwamoto F, Matsuoka K, Motobayashi M, et al. Prediction of disease activity of Crohn’s disease through fecal calprotectin evaluated by balloon-assisted endoscopy. J Gastroenterol Hepatol. 2018; 33:1984–1989.
Article6. Sipponen T, Savilahti E, Kolho KL, Nuutinen H, Turunen U, Färkkilä M. Crohn’s disease activity assessed by fecal calprotectin and lactoferrin: correlation with Crohn’s Disease Activity Index and endoscopic findings. Inflamm Bowel Dis. 2008; 14:40–46.
Article7. Zittan E, Kelly OB, Gralnek IM, Silverberg MS, Hillary Steinhart A. Fecal calprotectin correlates with active colonic inflammatory bowel disease but not with small intestinal Crohn’s disease activity. JGH Open. 2018; 2:201–206.
Article8. Mooiweer E, Fidder HH, Siersema PD, Laheij RJ, Oldenburg B. Fecal hemoglobin and calprotectin are equally effective in identifying patients with inflammatory bowel disease with active endoscopic inflammation. Inflamm Bowel Dis. 2014; 20:307–314.
Article9. Inokuchi T, Kato J, Hiraoka S, et al. Fecal immunochemical test versus fecal calprotectin for prediction of mucosal healing in Crohn’s disease. Inflamm Bowel Dis. 2016; 22:1078–1085.
Article10. Serada S, Fujimoto M, Terabe F, et al. Serum leucine-rich alpha-2 glycoprotein is a disease activity biomarker in ulcerative colitis. Inflamm Bowel Dis. 2012; 18:2169–2179.
Article11. Takenaka K, Kitazume Y, Kawamoto A, et al. Serum leucine-rich α2 glycoprotein: a novel biomarker for transmural inflammation in Crohn’s disease. Am J Gastroenterol. 2023; 118:1028–1035.
Article12. Kawamoto A, Takenaka K, Hibiya S, Ohtsuka K, Okamoto R, Watanabe M. Serum leucine-rich α2 glycoprotein: a novel biomarker for small bowel mucosal activity in Crohn’s disease. Clin Gastroenterol Hepatol. 2022; 20:e1196–e1200.
Article13. Kawamura T, Yamamura T, Nakamura M, et al. Accuracy of serum leucine-rich alpha-2 glycoprotein in evaluating endoscopic disease activity in Crohn’s disease. Inflamm Bowel Dis. 2023; 29:245–253.
Article14. Omori T, Sasaki Y, Koroku M, et al. Serum leucine-rich alpha-2 glycoprotein in quiescent Crohn’s disease as a potential surrogate marker for small-bowel ulceration detected by capsule endoscopy. J Clin Med. 2022; 11:2494.
Article15. Shinzaki S, Matsuoka K, Tanaka H, et al. Leucine-rich alpha-2 glycoprotein is a potential biomarker to monitor disease activity in inflammatory bowel disease receiving adalimumab: PLANET study. J Gastroenterol. 2021; 56:560–569.
Article16. Zubin G, Peter L. Predicting endoscopic Crohn’s disease activity before and after induction therapy in children: a comprehensive assessment of PCDAI, CRP, and fecal calprotectin. Inflamm Bowel Dis. 2015; 21:1386–1391.17. Bodelier AG, Jonkers D, van den Heuvel T, et al. High percentage of IBD patients with indefinite fecal calprotectin levels: additional value of a combination score. Dig Dis Sci. 2017; 62:465–472.
Article18. Ueno F, Matsui T, Matsumoto T, et al. Evidence-based clinical practice guidelines for Crohn’s disease, integrated with formal consensus of experts in Japan. J Gastroenterol. 2013; 48:31–72.
Article19. Takenaka K, Ohtsuka K, Kitazume Y, et al. Comparison of magnetic resonance and balloon enteroscopic examination of the small intestine in patients with Crohn’s disease. Gastroenterology. 2014; 147:334–342.
Article20. Takenaka K, Ohtsuka K, Kitazume Y, et al. Correlation of the endoscopic and magnetic resonance scoring systems in the deep small intestine in Crohn’s disease. Inflamm Bowel Dis. 2015; 21:1832–1838.
Article21. Ordás I, Rimola J, Alfaro I, et al. Development and validation of a simplified magnetic resonance index of activity for Crohn’s disease. Gastroenterology. 2019; 157:432–439.
Article22. Takenaka K, Fujii T, Suzuki K, et al. Small bowel healing detected by endoscopy in patients with Crohn’s disease after treatment with antibodies against tumor necrosis factor. Clin Gastroenterol Hepatol. 2020; 18:1545–1552.
Article23. Naka T, Fujimoto M. LRG is a novel inflammatory marker clinically useful for the evaluation of disease activity in rheumatoid arthritis and inflammatory bowel disease. Immunol Med. 2018; 41:62–67.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Balloon-Assisted Enteroscopy and Capsule Endoscopy in Suspected Small Bowel Crohn's Disease
- Efficacy of serum leucine-rich alpha-2 glycoprotein in predicting findings of Crohn’s disease small bowel lesion in capsule endoscopy
- Indications for Enteroscopy: Which Patients Should Be Recommended for Enteroscopy?
- Does Single Balloon Enteroscopy Have Similar Efficacy and Endoscopic Performance Compared with Double Balloon Enteroscopy?
- Usefulness of Serum Leucine-Rich Alpha-2 Glycoprotein as a Disease Activity Biomarker in Patients with Rheumatoid Arthritis