Int J Stem Cells.
2023 Nov;16(4):363-375. 10.15283/ijsc23045.
In Vivo Stem Cell Imaging Principles and Applications
- Affiliations
-
- 1Department of Anatomy, College of Medicine, Chung-Ang University, Seoul, Korea
- 2Department of Life Sciences, University of Seoul, Seoul, Korea
- 3Department of Life Science, Chung-Ang University, Seoul, Korea
- 4Stanford Cardiovascular Institute, Stanford University School of Medicine, Stanford, CA, USA
- KMID: 2548000
- DOI: http://doi.org/10.15283/ijsc23045
Abstract
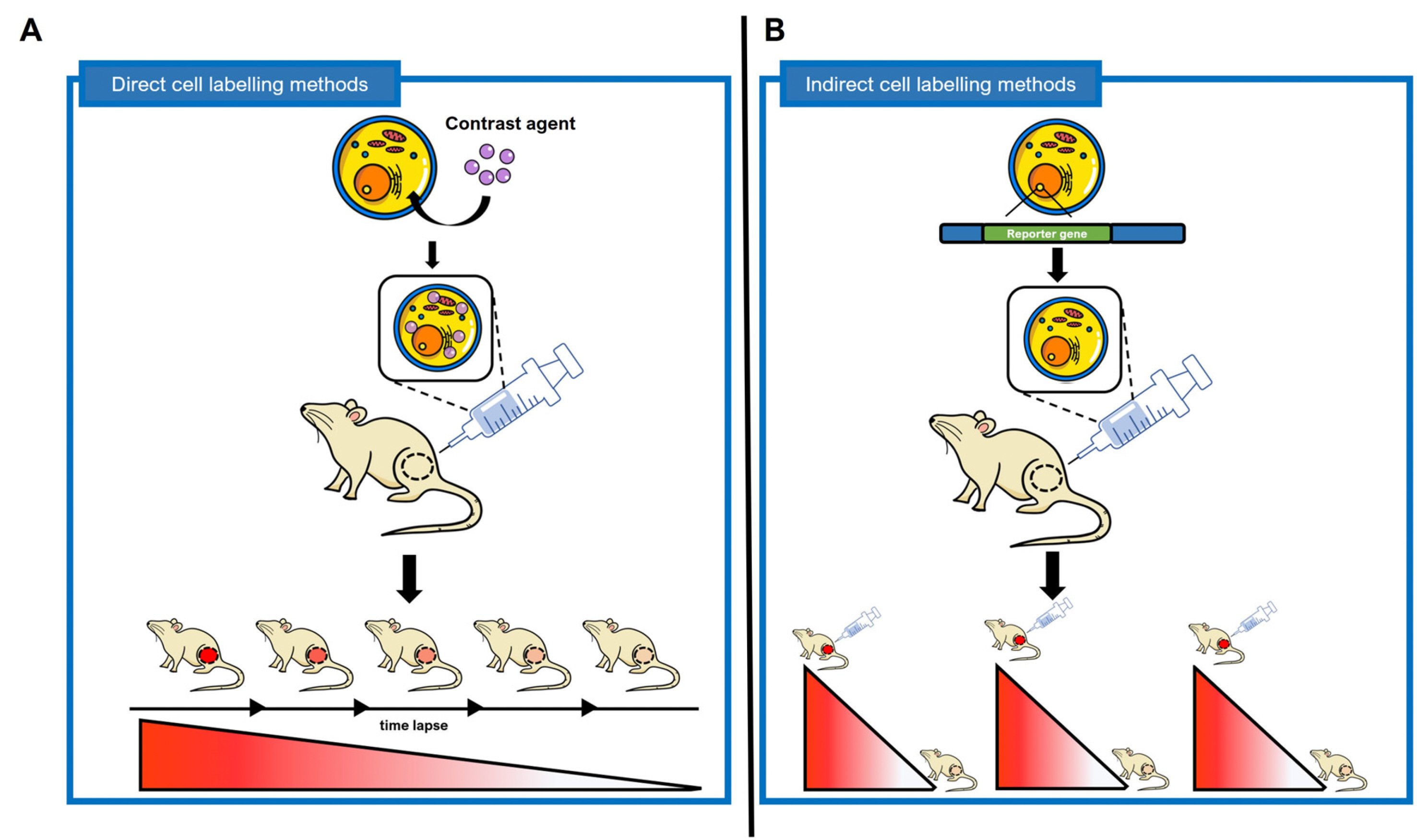
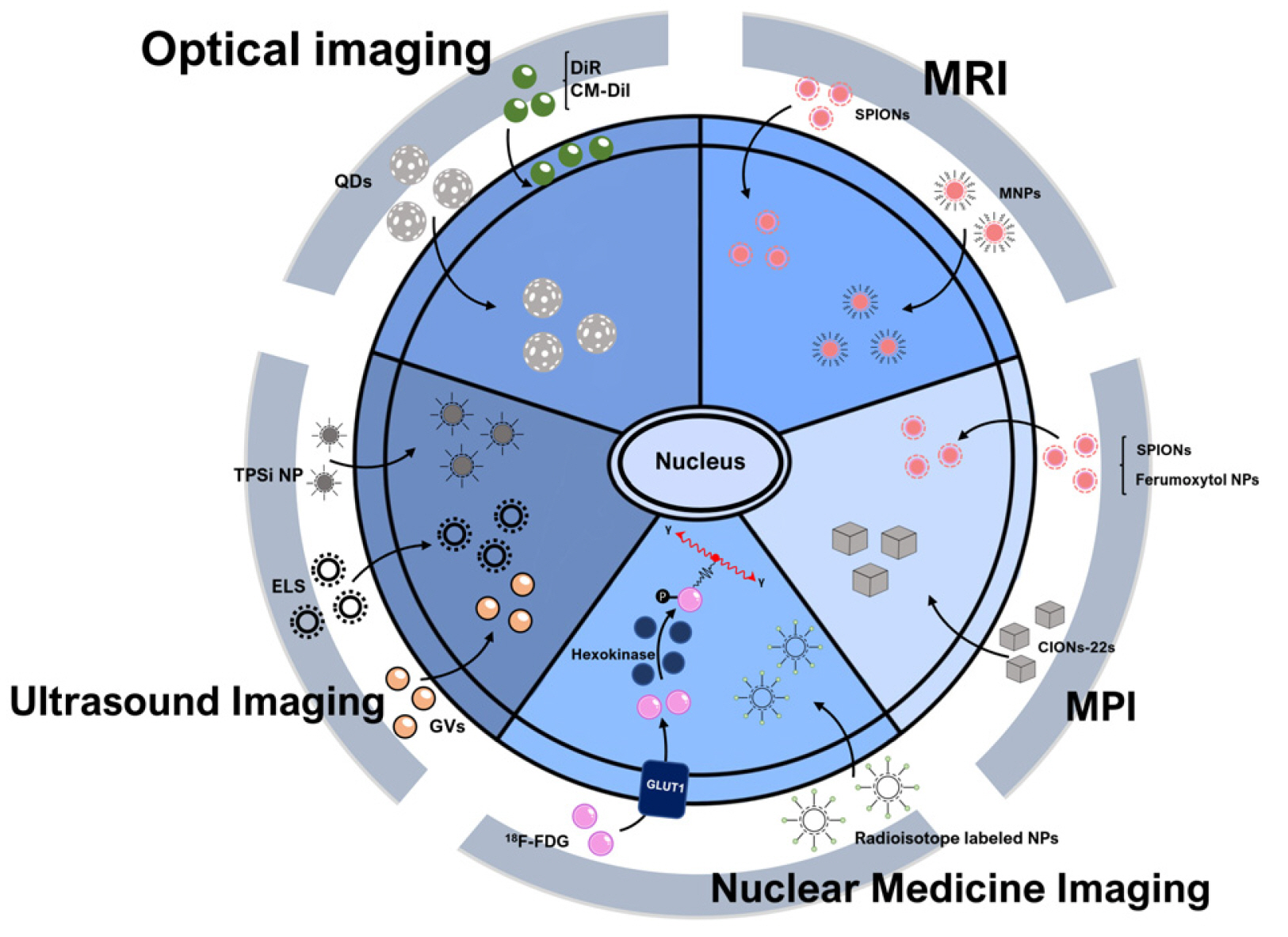
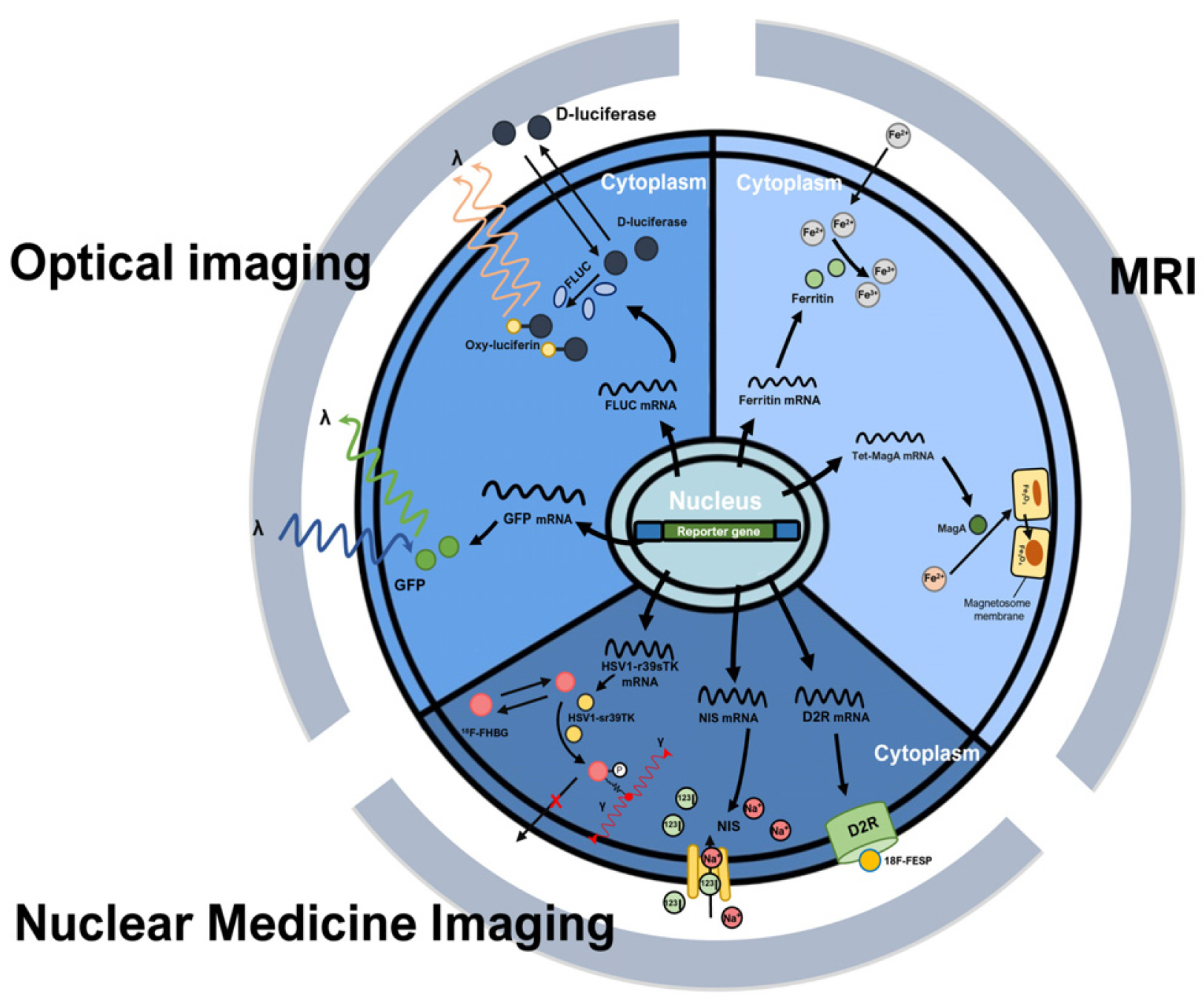
- Stem cells are the foundational cells for every organ and tissue in our body. Cell-based therapeutics using stem cells in regenerative medicine have received attracting attention as a possible treatment for various diseases caused by congenital defects. Stem cells such as induced pluripotent stem cells (iPSCs) as well as embryonic stem cells (ESCs), mesenchymal stem cells (MSCs), and neuroprogenitors stem cells (NSCs) have recently been studied in various ways as a cell-based therapeutic agent. When various stem cells are transplanted into a living body, they can differentiate and perform complex functions. For stem cell transplantation, it is essential to determine the suitability of the stem cell-based treatment by evaluating the origin of stem, the route of administration, In vivo bio-distribution, transplanted cell survival, function, and mobility. Currently, these various stem cells are being imaged In vivo through various molecular imaging methods. Various imaging modalities such as optical imaging, magnetic resonance imaging (MRI), ultrasound (US), positron emission tomography (PET), and single-photon emission computed tomography (SPECT) have been introduced for the application of various stem cell imaging. In this review, we discuss the principles and recent advances of In vivo molecular imaging for application of stem cell research.
Keyword
Figure
Reference
-
References
1. Mason C, Dunnill P. 2008; A brief definition of regenerative medicine. Regen Med. 3:1–5. DOI: 10.2217/17460751.3.1.1. PMID: 18154457.
Article2. Mahla RS. 2016; Stem cells applications in regenerative medicine and disease therapeutics. Int J Cell Biol. 2016:6940283. DOI: 10.1155/2016/6940283. PMID: 27516776. PMCID: PMC4969512. PMID: 299fd671a7944b77afff0653031efe6f.
Article3. Leferink AM, Chng YC, van Blitterswijk CA, Moroni L. 2015; Distribution and viability of fetal and adult human bone marrow stromal cells in a biaxial rotating vessel bioreactor after seeding on polymeric 3D additive manufactured scaffolds. Front Bioeng Biotechnol. 3:169. DOI: 10.3389/fbioe.2015.00169. PMID: 26557644. PMCID: PMC4617101. PMID: 084e7c5cf3844c6fba70e5b7514f6d31.
Article4. Gubareva EA, Sjöqvist S, Gilevich IV, et al. 2016; Orthotopic transplantation of a tissue engineered diaphragm in rats. Biomaterials. 77:320–335. DOI: 10.1016/j.biomaterials.2015.11.020. PMID: 26618750.
Article5. Garzón I, Pérez-Köhler B, Garrido-Gómez J, et al. 2012; Evalua-tion of the cell viability of human Wharton's jelly stem cells for use in cell therapy. Tissue Eng Part C Methods. 18:408–419. DOI: 10.1089/ten.tec.2011.0508. PMID: 22166141. PMCID: PMC3358099.
Article6. Thompson PA, Perera T, Marin D, et al. 2016; Double umbilical cord blood transplant is effective therapy for relapsed or refractory Hodgkin lymphoma. Leuk Lymphoma. 57:1607–1615. DOI: 10.3109/10428194.2015.1105370. PMID: 26472485. PMCID: PMC6545894.
Article7. Boehm-Sturm P, Mengler L, Wecker S, Hoehn M, Kallur T. 2011; In vivo tracking of human neural stem cells with 19F magnetic resonance imaging. PLoS One. 6:e29040. DOI: 10.1371/journal.pone.0029040. PMID: 22216163. PMCID: PMC3247235. PMID: 6bd578a4ce194aa4adf19dde78d8d910.
Article8. Hong H, Yang Y, Zhang Y, Cai W. 2010; Non-invasive cell tracking in cancer and cancer therapy. Curr Top Med Chem. 10:1237–1248. DOI: 10.2174/156802610791384234. PMID: 20388105. PMCID: PMC2916057.9. Wang L, Maslov K, Wang LV. 2013; Single-cell label-free photoacoustic flowoxigraphy in vivo. Proc Natl Acad Sci U S A. 110:5759–5764. DOI: 10.1073/pnas.1215578110. PMID: 23536296. PMCID: PMC3625281.
Article10. Murakami T, Chun N. 2009. Dec. 17. Bioluminescent imaging and organ-specific metastasis of human cancer cells [Internet]. The International Society for Optics and Pho-tonics (SPIE);Bellingham: Available from: https://spie.org/news/2501-bioluminescent-imaging-and-organ-specific-metastasis-of-human-cancer-cells?SSO=1. cited 2023 Jun 1. DOI: 10.1117/2.1200912.002501.11. Iwano S, Sugiyama M, Hama H, et al. 2018; Single-cell biolu-minescence imaging of deep tissue in freely moving ani-mals. Science. 359:935–939. DOI: 10.1126/science.aaq1067. PMID: 29472486.
Article12. Herynek V, Turnovcová K, Gálisová A, et al. 2019; Manganese-zinc ferrites: safe and efficient nanolabels for cell imaging and tracking in vivo. ChemistryOpen. 8:155–165. DOI: 10.1002/open.201800261. PMID: 30740290. PMCID: PMC6356160.
Article13. Man F, Lim L, Volpe A, et al. 2019; In vivo PET tracking of 89Zr-Labeled Vγ9Vδ2 T cells to mouse xenograft breast tumors activated with liposomal alendronate. Mol Ther. 27:219–229. DOI: 10.1016/j.ymthe.2018.10.006. PMID: 30429045. PMCID: PMC6318719.
Article14. Kircher MF, Gambhir SS, Grimm J. 2011; Noninvasive cell-tracking methods. Nat Rev Clin Oncol. 8:677–688. DOI: 10.1038/nrclinonc.2011.141. PMID: 21946842.
Article15. Youn H, Hong KJ. 2012; In vivo non invasive molecular imaging for immune cell tracking in small animals. Immune Netw. 12:223–229. DOI: 10.4110/in.2012.12.6.223. PMID: 23396713. PMCID: PMC3566416.
Article16. Concilio SC, Russell SJ, Peng KW. 2021; A brief review of reporter gene imaging in oncolytic virotherapy and gene therapy. Mol Ther Oncolytics. 21:98–109. DOI: 10.1016/j.omto.2021.03.006. PMID: 33981826. PMCID: PMC8065251.
Article17. Martelli C, Lo Dico A, Diceglie C, Lucignani G, Ottobrini L. 2016; Optical imaging probes in oncology. Oncotarget. 7:48753–48787. DOI: 10.18632/oncotarget.9066. PMID: 27145373. PMCID: PMC5217050.
Article18. Michalet X, Pinaud FF, Bentolila LA, et al. 2005; Quantum dots for live cells, in vivo imaging, and diagnostics. Science. 307:538–544. DOI: 10.1126/science.1104274. PMID: 15681376. PMCID: PMC1201471.
Article19. Kalchenko V, Shivtiel S, Malina V, et al. 2006; Use of lipophilic near-infrared dye in whole-body optical imaging of hematopoietic cell homing. J Biomed Opt. 11:050507. DOI: 10.1117/1.2364903. PMID: 17092148.
Article20. Seleverstov O, Zabirnyk O, Zscharnack M, et al. 2006; Quantum dots for human mesenchymal stem cells labeling. A size-dependent autophagy activation. Nano Lett. 6:2826–2832. DOI: 10.1021/nl0619711. PMID: 17163713.
Article21. Lin S, Xie X, Patel MR, et al. 2007; Quantum dot imaging for embryonic stem cells. BMC Biotechnol. 7:67. DOI: 10.1186/1472-6750-7-67. PMID: 17925032. PMCID: PMC2174930. PMID: a60bdb30a4f34ab690151668ec777612.
Article22. Ruan J, Song H, Li C, et al. 2012; DiR-labeled embryonic stem cells for targeted imaging of in vivo gastric cancer cells. Theranostics. 2:618–628. DOI: 10.7150/thno.4561. PMID: 22768029. PMCID: PMC3388594.
Article23. Lassailly F, Griessinger E, Bonnet D. 2010; "Microenvironmental contaminations" induced by fluorescent lipophilic dyes used for noninvasive in vitro and in vivo cell tracking. Blood. 115:5347–5354. DOI: 10.1182/blood-2009-05-224030. PMID: 20215639.
Article24. Sun S, Chen G, Xu M, Qiao Y, Zheng S. 2013; Differentiation and migration of bone marrow mesenchymal stem cells transplanted through the spleen in rats with portal hyper-tension. PLoS One. 8:e83523. DOI: 10.1371/journal.pone.0083523. PMID: 24340101. PMCID: PMC3858351. PMID: b515344b2b3748278a21284a018659eb.
Article25. Akhan E, Tuncel D, Akcali KC. 2015; Nanoparticle labeling of bone marrow-derived rat mesenchymal stem cells: their use in differentiation and tracking. Biomed Res Int. 2015:298430. DOI: 10.1155/2015/298430. PMID: 25654092. PMCID: PMC4310257.
Article26. Hu SL, Lu PG, Zhang LJ, et al. 2012; In vivo magnetic resonance imaging tracking of SPIO-labeled human umbilical cord mesenchymal stem cells. J Cell Biochem. 113:1005–1012. DOI: 10.1002/jcb.23432. PMID: 22065605.
Article27. Zare S, Mehrabani D, Jalli R, et al. 2019; MRI-tracking of dental pulp stem cells in vitro and in vivo using dextran-coated superparamagnetic iron oxide nanoparticles. J Clin Med. 8:1418. DOI: 10.3390/jcm8091418. PMID: 31505807. PMCID: PMC6780915. PMID: 45f4511c93a54217b7f18018df138c99.
Article28. Pang P, Wu C, Gong F, et al. 2015; Nanovector for gene transfection and MR imaging of mesenchymal stem cells. J Biomed Nanotechnol. 11:644–656. DOI: 10.1166/jbn.2015.1967. PMID: 26310071.
Article29. Kim TH, Kim JK, Shim W, Kim SY, Park TJ, Jung JY. 2010; Tracking of transplanted mesenchymal stem cells labeled with fluorescent magnetic nanoparticle in liver cirrhosis rat model with 3-T MRI. Magn Reson Imaging. 28:1004–1013. DOI: 10.1016/j.mri.2010.03.047. PMID: 20663626.
Article30. Walczak P, Kedziorek DA, Gilad AA, Lin S, Bulte JW. 2005; Instant MR labeling of stem cells using magnetoelectro-poration. Magn Reson Med. 54:769–774. DOI: 10.1002/mrm.20701. PMID: 16161115.
Article31. Zhou XY, Tay ZW, Chandrasekharan P, et al. 2018; Magnetic particle imaging for radiation-free, sensitive and high-contrast vascular imaging and cell tracking. Curr Opin Chem Biol. 45:131–138. DOI: 10.1016/j.cbpa.2018.04.014. PMID: 29754007. PMCID: PMC6500458.
Article32. Du Y, Lai PT, Leung CH, Pong PW. 2013; Design of superparamagnetic nanoparticles for magnetic particle imaging (MPI). Int J Mol Sci. 14:18682–18710. DOI: 10.3390/ijms140918682. PMID: 24030719. PMCID: PMC3794803. PMID: 282299672aba4f159b667712a34d33bb.
Article33. Zheng B, von See MP, Yu E, et al. 2016; Quantitative magnetic particle imaging monitors the transplantation, biodistri-bution, and clearance of stem cells in vivo. Theranostics. 6:291–301. DOI: 10.7150/thno.13728. PMID: 26909106. PMCID: PMC4737718.
Article34. Sehl OC, Makela AV, Hamilton AM, Foster PJ. 2019; Trimodal cell tracking in vivo: combining iron- and fluorine-based magnetic resonance imaging with magnetic particle imaging to monitor the delivery of mesenchymal stem cells and the ensuing inflammation. Tomography. 5:367–376. DOI: 10.18383/j.tom.2019.00020. PMID: 31893235. PMCID: PMC6935990.
Article35. Wang Q, Ma X, Liao H, et al. 2020; Artificially engineered cubic iron oxide nanoparticle as a high-performance magnetic particle imaging tracer for stem cell tracking. ACS Nano. 14:2053–2062. DOI: 10.1021/acsnano.9b08660. PMID: 31999433.
Article36. Uenomachi M, Takahashi M, Shimazoe K, et al. 2021; Simulta-neous in vivo imaging with PET and SPECT tracers using a Compton-PET hybrid camera. Sci Rep. 11:17933. DOI: 10.1038/s41598-021-97302-7. PMID: 34504184. PMCID: PMC8429650. PMID: 5c1dd89e6a52433cb78a04570ae4efa4.
Article37. Tong L, Zhao H, He Z, Li Z. Erondu OF, editor. 2013. Current perspectives on molecular imaging for tracking stem cell therapy. Medical Imaging in Clinical Practice. InTech;p. 63–79. DOI: 10.5772/53028.38. Jiang W, Chalich Y, Deen MJ. 2019; Sensors for positron emission tomography applications. Sensors (Basel). 19:5019. DOI: 10.3390/s19225019. PMID: 31744258. PMCID: PMC6891456. PMID: 0908d5f1944248c7a52b4b240891263d.
Article39. Kapoor V, McCook BM, Torok FS. 2004; An introduction to PET-CT imaging. Radiographics. 24:523–543. DOI: 10.1148/rg.242025724. PMID: 15026598.
Article40. Cook GJ, Wegner EA, Fogelman I. 2004; Pitfalls and artifacts in 18FDG PET and PET/CT oncologic imaging. Semin Nucl Med. 34:122–133. DOI: 10.1053/j.semnuclmed.2003.12.003. PMID: 15031812.
Article41. Nose N, Nogami S, Koshino K, et al. 2021; [18F]FDG-labelled stem cell PET imaging in different route of administrations and multiple animal species. Sci Rep. 11:10896. DOI: 10.1038/s41598-021-90383-4. PMID: 34035416. PMCID: PMC8149709. PMID: 9212afd2f67542e299355bc54cce9b67.
Article42. Yao M, Shi X, Zuo C, et al. 2020; Engineering of SPECT/photoacoustic imaging/antioxidative stress triple-function nanoprobe for advanced mesenchymal stem cell therapy of cerebral ischemia. ACS Appl Mater Interfaces. 12:37885–37895. DOI: 10.1021/acsami.0c10500. PMID: 32806884.
Article43. Wang J, Jokerst JV. 2016; Stem cell imaging: tools to improve cell delivery and viability. Stem Cells Int. 2016:9240652. DOI: 10.1155/2016/9240652. PMID: 26880997. PMCID: PMC4736428. PMID: 8f1af55894fb4a65970b22ba5e52b753.
Article44. Zaw Thin M, Allan H, Bofinger R, et al. 2020; Multi-modal imaging probe for assessing the efficiency of stem cell delivery to orthotopic breast tumours. Nanoscale. 12:16570–16585. DOI: 10.1039/D0NR03237A. PMID: 32749427. PMCID: PMC7586303.
Article45. Chen F, Jokerst JV. 2020; Stem cell tracking with nanoparticle-based ultrasound contrast agents. Methods Mol Biol. 2126:141–153. DOI: 10.1007/978-1-0716-0364-2_13. PMID: 32112386. PMCID: PMC8045894.
Article46. Abou-Elkacem L, Bachawal SV, Willmann JK. 2015; Ultrasound molecular imaging: moving toward clinical translation. Eur J Radiol. 84:1685–1693. DOI: 10.1016/j.ejrad.2015.03.016. PMID: 25851932. PMCID: PMC4545409.
Article47. James ML, Gambhir SS. 2012; A molecular imaging primer: modalities, imaging agents, and applications. Physiol Rev. 92:897–965. DOI: 10.1152/physrev.00049.2010. PMID: 22535898.
Article48. Xu C, Feng Q, Ning P, Li Z, Qin Y, Cheng Y. 2021; Recent advances on nanoparticle-based imaging contrast agents for in vivo stem cell tracking. Mat Matters. 16:2.49. Chen F, Ma M, Wang J, et al. 2017; Exosome-like silica nanoparticles: a novel ultrasound contrast agent for stem cell imaging. Nanoscale. 9:402–411. DOI: 10.1039/C6NR08177K. PMID: 27924340. PMCID: PMC5179289.
Article50. Qi S, Zhang P, Ma M, et al. 2019; Cellular internalization-in-duced aggregation of porous silicon nanoparticles for ultrasound imaging and protein-mediated protection of stem cells. Small. 15:e1804332. DOI: 10.1002/smll.201804332. PMID: 30488562.
Article51. Gong Z, He Y, Zhou M, et al. 2022; Ultrasound imaging tracking of mesenchymal stem cells intracellularly labeled with biosynthetic gas vesicles for treatment of rheumatoid arthritis. Theranostics. 12:2370–2382. DOI: 10.7150/thno.66905. PMID: 35265215. PMCID: PMC8899585.
Article52. Gawne PJ, Man F, Blower PJ. T M de Rosales R. 2022; Direct cell radiolabeling for in vivo cell tracking with PET and SPECT imaging. Chem Rev. 122:10266–10318. DOI: 10.1021/acs.chemrev.1c00767. PMID: 35549242. PMCID: PMC9185691.
Article53. Kang JH, Chung JK. 2008; Molecular-genetic imaging based on reporter gene expression. J Nucl Med. 49 Suppl 2:164S–179S. DOI: 10.2967/jnumed.107.045955. PMID: 18523072.
Article54. Müller-Taubenberger A. 2006; Application of fluorescent protein tags as reporters in live-cell imaging studies. Methods Mol Biol. 346:229–246. DOI: 10.1385/1-59745-144-4:229. PMID: 16957294.
Article55. Chudakov DM, Lukyanov S, Lukyanov KA. 2005; Fluorescent proteins as a toolkit for in vivo imaging. Trends Biotechnol. 23:605–613. DOI: 10.1016/j.tibtech.2005.10.005. PMID: 16269193.
Article56. Ke CC, Liu RS, Suetsugu A, et al. 2013; In vivo fluorescence imaging reveals the promotion of mammary tumorigenesis by mesenchymal stromal cells. PLoS One. 8:e69658. DOI: 10.1371/journal.pone.0069658. PMID: 23936067. PMCID: PMC3723858. PMID: 183006890e43408bb9c248320eb37121.
Article57. Chen Q, Wang X, Wu H, et al. 2016; Establishment of a dual-color fluorescence tracing orthotopic transplantation model of hepatocellular carcinoma. Mol Med Rep. 13:762–768. DOI: 10.3892/mmr.2015.4624. PMID: 26647736. PMCID: PMC4686090.
Article58. Troy T, Jekic-McMullen D, Sambucetti L, Rice B. 2004; Quan-titative comparison of the sensitivity of detection of fluorescent and bioluminescent reporters in animal models. Mol Imaging. 3:9–23. DOI: 10.1162/153535004773861688. PMID: 15142408.
Article59. Hoffman RM. 2015; Application of GFP imaging in cancer. Lab Invest. 95:432–452. DOI: 10.1038/labinvest.2014.154. PMID: 25686095. PMCID: PMC4383682.
Article60. Ezzat T, Dhar DK, Malago M, Olde Damink SW. 2012; Dynamic tracking of stem cells in an acute liver failure model. World J Gastroenterol. 18:507–516. DOI: 10.3748/wjg.v18.i6.507. PMID: 22363116. PMCID: PMC3280395.
Article61. Bhaumik S, Lewis XZ, Gambhir SS. 2004; Optical imaging of Renilla luciferase, synthetic Renilla luciferase, and firefly luciferase reporter gene expression in living mice. J Biomed Opt. 9:578–586. DOI: 10.1117/1.1647546. PMID: 15189096.
Article62. Badr CE, Tannous BA. 2011; Bioluminescence imaging: progress and applications. Trends Biotechnol. 29:624–633. DOI: 10.1016/j.tibtech.2011.06.010. PMID: 21788092. PMCID: PMC4314955.
Article63. Iyer M, Sato M, Johnson M, Gambhir SS, Wu L. 2005; Applications of molecular imaging in cancer gene therapy. Curr Gene Ther. 5:607–618. DOI: 10.2174/156652305774964695. PMID: 16457650.
Article64. Subramaniam D, Natarajan G, Ramalingam S, et al. 2008; Trans-lation inhibition during cell cycle arrest and apoptosis: Mcl-1 is a novel target for RNA binding protein CUGBP2. Am J Physiol Gastrointest Liver Physiol. 294:G1025–G1032. DOI: 10.1152/ajpgi.00602.2007. PMID: 18292181.
Article65. van der Bogt KE, Sheikh AY, Schrepfer S, et al. 2008; Compa-rison of different adult stem cell types for treatment of myocardial ischemia. Circulation. 118(14 Suppl):S121–S129. DOI: 10.1161/CIRCULATIONAHA.107.759480. PMID: 18824743. PMCID: PMC3657517.
Article66. Preda MB, Neculachi CA, Fenyo IM, et al. 2021; Short lifespan of syngeneic transplanted MSC is a consequence of in vivo apoptosis and immune cell recruitment in mice. Cell Death Dis. 12:566. DOI: 10.1038/s41419-021-03839-w. PMID: 34075029. PMCID: PMC8169682. PMID: c870d193b4064f9b8e3320f0af18e8c8.
Article67. Kalimuthu S, Oh JM, Gangadaran P, et al. 2017; Genetically engineered suicide gene in mesenchymal stem cells using a Tet-On system for anaplastic thyroid cancer. PLoS One. 12:e0181318. DOI: 10.1371/journal.pone.0181318. PMID: 28727740. PMCID: PMC5519161. PMID: 9ca441cfcdb04debb51044b18b5f4cb3.
Article68. Gao T, Wang P, Gong T, et al. 2022; Reporter genes for brain imaging using MRI, SPECT and PET. Int J Mol Sci. 23:8443. DOI: 10.3390/ijms23158443. PMID: 35955578. PMCID: PMC9368793. PMID: 7bab98650d014c0ca77d012a6e08ee5b.
Article69. Kraitchman DL, Bulte JW. 2008; Imaging of stem cells using MRI. Basic Res Cardiol. 103:105–113. DOI: 10.1007/s00395-008-0704-5. PMID: 18324366. PMCID: PMC3037795.
Article70. Yang C, Tian R, Liu T, Liu G. 2016; MRI reporter genes for noninvasive molecular imaging. Molecules. 21:580. DOI: 10.3390/molecules21050580. PMID: 27213309. PMCID: PMC6273230. PMID: 022ed8056b1f4a7fb5ef845aea873180.
Article71. Gilad AA, Ziv K, McMahon MT, van Zijl PC, Neeman M, Bulte JW. 2008; MRI reporter genes. J Nucl Med. 49:1905–1908. DOI: 10.2967/jnumed.108.053520. PMID: 18997049. PMCID: PMC2730187.
Article72. Weissleder R, Moore A, Mahmood U, et al. 2000; In vivo magnetic resonance imaging of transgene expression. Nat Med. 6:351–354. DOI: 10.1038/73219. PMID: 10700241.
Article73. Ichikawa T, Högemann D, Saeki Y, et al. 2002; MRI of transgene expression: correlation to therapeutic gene expression. Neoplasia. 4:523–530. DOI: 10.1038/sj.neo.7900266. PMID: 12407446. PMCID: PMC1503666. PMID: c62c2c31b86b4f0b85a5e26f2452e144.
Article74. Cohen B, Dafni H, Meir G, Harmelin A, Neeman M. 2005; Ferritin as an endogenous MRI reporter for noninvasive imaging of gene expression in C6 glioma tumors. Neoplasia. 7:109–117. DOI: 10.1593/neo.04436. PMID: 15802016. PMCID: PMC1501126. PMID: 75f7fd3e1feb45a68985f716d470fa01.
Article75. Cohen B, Ziv K, Plaks V, et al. 2007; MRI detection of transcriptional regulation of gene expression in transgenic mice. Nat Med. 13:498–503. DOI: 10.1038/nm1497. PMID: 17351627.
Article76. Genove G, DeMarco U, Xu H, Goins WF, Ahrens ET. 2005; A new transgene reporter for in vivo magnetic resonance ima-ging. Nat Med. 11:450–454. DOI: 10.1038/nm1208. PMID: 15778721.
Article77. Deans AE, Wadghiri YZ, Bernas LM, Yu X, Rutt BK, Turnbull DH. 2006; Cellular MRI contrast via coexpression of transferrin receptor and ferritin. Magn Reson Med. 56:51–59. DOI: 10.1002/mrm.20914. PMID: 16724301. PMCID: PMC4079558.
Article78. Cao M, Mao J, Duan X, et al. 2018; In vivo tracking of the tropism of mesenchymal stem cells to malignant gliomas using reporter gene-based MR imaging. Int J Cancer. 142:1033–1046. DOI: 10.1002/ijc.31113. PMID: 29047121.
Article79. Sun J, Huang J, Bao G, et al. 2021; MRI detection of the malignant transformation of stem cells through reporter gene expression driven by a tumor-specific promoter. Stem Cell Res Ther. 12:284. DOI: 10.1186/s13287-021-02359-w. PMID: 33980305. PMCID: PMC8117323. PMID: da18664f679441d69bfb4fc85c4dfd77.
Article80. Cho IK, Moran SP, Paudyal R, et al. 2014; Longitudinal monitoring of stem cell grafts in vivo using magnetic resonance imaging with inducible maga as a genetic reporter. Thera-nostics. 4:972–989. DOI: 10.7150/thno.9436. PMID: 25161700. PMCID: PMC4143941.
Article81. Gambhir SS. 2002; Molecular imaging of cancer with positron emission tomography. Nat Rev Cancer. 2:683–693. DOI: 10.1038/nrc882. PMID: 12209157.
Article82. Youn H, Hong KJ. 2012; In vivo noninvasive small animal molecular imaging. Osong Public Health Res Perspect. 3:48–59. DOI: 10.1016/j.phrp.2012.02.002. PMID: 24159487. PMCID: PMC3738683.83. Tjuvajev JG, Doubrovin M, Akhurst T, et al. 2002; Comparison of radiolabeled nucleoside probes (FIAU, FHBG, and FHPG) for PET imaging of HSV1-tk gene expression. J Nucl Med. 43:1072–1083. PMID: 12163634.84. Peñuelas I, Haberkorn U, Yaghoubi S, Gambhir SS. 2005; Gene therapy imaging in patients for oncological applications. Eur J Nucl Med Mol Imaging. 32 Suppl 2:S384–S403. DOI: 10.1007/s00259-005-1928-3. PMID: 16180032.
Article85. Liang Q, Satyamurthy N, Barrio JR, et al. 2001; Noninvasive, quantitative imaging in living animals of a mutant dopamine D2 receptor reporter gene in which ligand binding is uncoupled from signal transduction. Gene Ther. 8:1490–1498. DOI: 10.1038/sj.gt.3301542. PMID: 11593362.
Article86. Schönitzer V, Haasters F, Käsbauer S, et al. 2014; In vivo mesenchymal stem cell tracking with PET using the dopamine type 2 receptor and 18F-fallypride. J Nucl Med. 55:1342–1347. DOI: 10.2967/jnumed.113.134775. PMID: 25024426.
Article87. Kitzberger C, Spellerberg R, Morath V, et al. 2022; The sodium iodide symporter (NIS) as theranostic gene: its emerging role in new imaging modalities and non-viral gene therapy. EJNMMI Res. 12:25. DOI: 10.1186/s13550-022-00888-w. PMID: 35503582. PMCID: PMC9065223. PMID: 7399ed72a2d84c20be101a3d6215c0fb.
Article88. Spitzweg C, Bible KC, Hofbauer LC, Morris JC. 2014; Advanced radioiodine-refractory differentiated thyroid cancer: the sodium iodide symporter and other emerging therapeutic targets. Lancet Diabetes Endocrinol. 2:830–842. DOI: 10.1016/S2213-8587(14)70051-8. PMID: 24898835.
Article89. Gu E, Chen WY, Gu J, Burridge P, Wu JC. 2012; Molecular imaging of stem cells: tracking survival, biodistribution, tumorigenicity, and immunogenicity. Theranostics. 2:335–345. DOI: 10.7150/thno.3666. PMID: 22509197. PMCID: PMC3326720.
Article90. Terrovitis J, Kwok KF, Lautamäki R, et al. 2008; Ectopic expression of the sodium-iodide symporter enables imaging of transplanted cardiac stem cells in vivo by single-photon emission computed tomography or positron emission tomo-graphy. J Am Coll Cardiol. 52:1652–1660. DOI: 10.1016/j.jacc.2008.06.051. PMID: 18992656. PMCID: PMC4980098.
Article91. Dwyer RM, Ryan J, Havelin RJ, et al. 2011; Mesenchymal Stem Cell-mediated delivery of the sodium iodide symporter supports radionuclide imaging and treatment of breast cancer. Stem Cells. 29:1149–1157. DOI: 10.1002/stem.665. PMID: 21608083. PMCID: PMC3998644.
Article92. Sharif-Paghaleh E, Sunassee K, Tavaré R, et al. 2011; In vivo SPECT reporter gene imaging of regulatory T cells. PLoS One. 6:e25857. DOI: 10.1371/journal.pone.0025857. PMID: 22043296. PMCID: PMC3197183. PMID: 0ea4ae1396b74ba491e13c829f5520f6.
Article93. Price DN, McBride AA, Anton M, et al. 2016; Longitudinal assessment of lung cancer progression in mice using the sodium iodide symporter reporter gene and SPECT/CT ima-ging. PLoS One. 11:e0169107. DOI: 10.1371/journal.pone.0169107. PMID: 28036366. PMCID: PMC5201271. PMID: d6246baf7cc9492692dea8a85eb76f80.
Article94. Knoop K, Kolokythas M, Klutz K, et al. 2011; Image-guided, tumor stroma-targeted 131I therapy of hepatocellular cancer after systemic mesenchymal stem cell-mediated NIS gene delivery. Mol Ther. 19:1704–1713. DOI: 10.1038/mt.2011.93. PMID: 21587211. PMCID: PMC3182369.
Article95. Conrad C, Hüsemann Y, Niess H, et al. 2011; Linking transgene expression of engineered mesenchymal stem cells and angiopoietin-1-induced differentiation to target cancer angio-genesis. Ann Surg. 253:566–571. DOI: 10.1097/SLA.0b013e3181fcb5d8. PMID: 21169810.
Article