Cancer Res Treat.
2023 Oct;55(4):1355-1362. 10.4143/crt.2023.271.
Intensified First Cycle of Rituximab Plus Eight Cycles of Cyclophosphamide, Doxorubicin, Vincristine, and Prednisolone with Rituximab Chemotherapy for Advanced-Stage or Bulky Diffuse Large B-Cell Lymphoma: A Multicenter Phase II Consortium for Improving Survival of Lymphoma (CISL) Study
- Affiliations
-
- 1Division of Hematology, Department of Internal Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- 2Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 3Hematology-Oncology Clinic, National Cancer Center, Goyang, Korea
- 4Department of Hematology/Oncology, Chonnam National University Hwasun Hospital, Hwasun, Korea
- 5Department of Internal Medicine, Daegu Catholic University Medical Center, Daegu, Korea
- 6Department of Internal Medicine, Hallym University Sacred Heart Hospital, Anyang, Korea
- 7Departments of Internal Medicine, Gachon University Gil Medical Center, Incheon, Korea
- 8Department of Internal Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea
- 9Department of Internal Medicine, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- 10Department of Internal Medicine, Jeonbuk National University Medical School, Jeonju, Korea
- 11Department of Internal Medicine, Korea University College of Medicine, Seoul, Korea
- 12Department of Internal Medicine, Yeungnam University College of Medicine, Daegu, Korea
- 13Department of Internal Medicine, Dong-A University College of Medicine, Busan, Korea
- 14Department of Internal Medicine, Korea Cancer Center Hospital, Korea Institute of Radiological and Medical Sciences, Seoul, Korea
- 15Department of Internal Medicine, Veterans Health Service Medical Center, Seoul, Korea
- 16Department of Internal Medicine, Wonkwang University School of Medicine, Iksan, Korea
- 17Department of Hematology-Oncology, Ajou University School of Medicine, Suwon, Korea
- 18Department of Internal Medicine, Ewha Womans University School of Medicine, Seoul, Korea
- 19Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2547809
- DOI: http://doi.org/10.4143/crt.2023.271
Abstract
- Purpose
This phase II, open-label, multicenter study aimed to investigate the efficacy and safety of a rituximab intensification for the 1st cycle with every 21-day of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP-21) among patients with previously untreated advanced-stage or bulky diffuse large B-cell lymphoma (DLBCL).
Materials and Methods
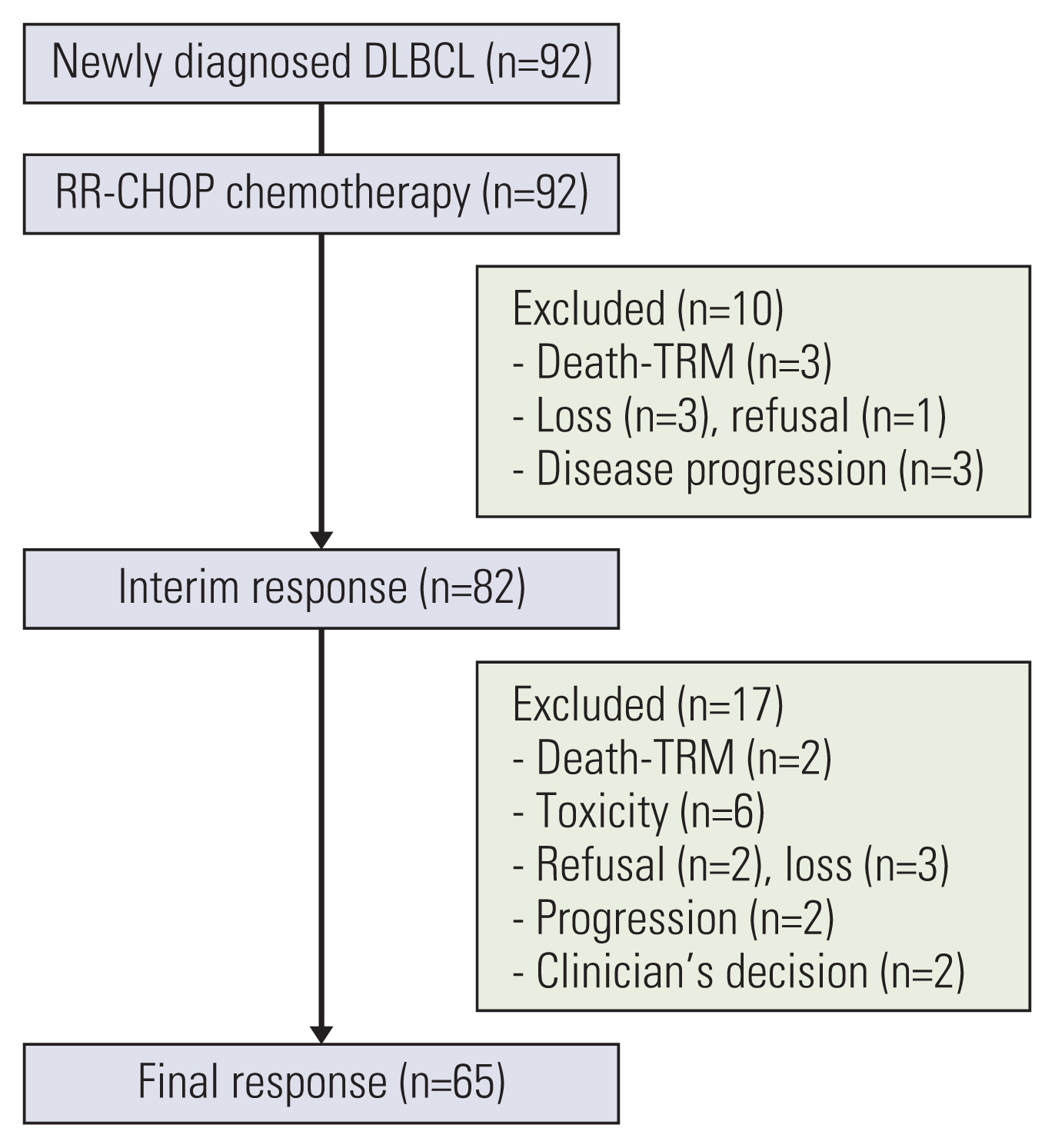
Ninety-two patients with stage III/IV or bulky DLBCL from 21 institutions were administered 8 cycles of R-CHOP-21 with an additional one dose of rituximab intensification on day 0 of the 1st cycle (RR-CHOP). The primary endpoint was a complete response (CR) rate after 3 cycles of chemotherapy.
Results
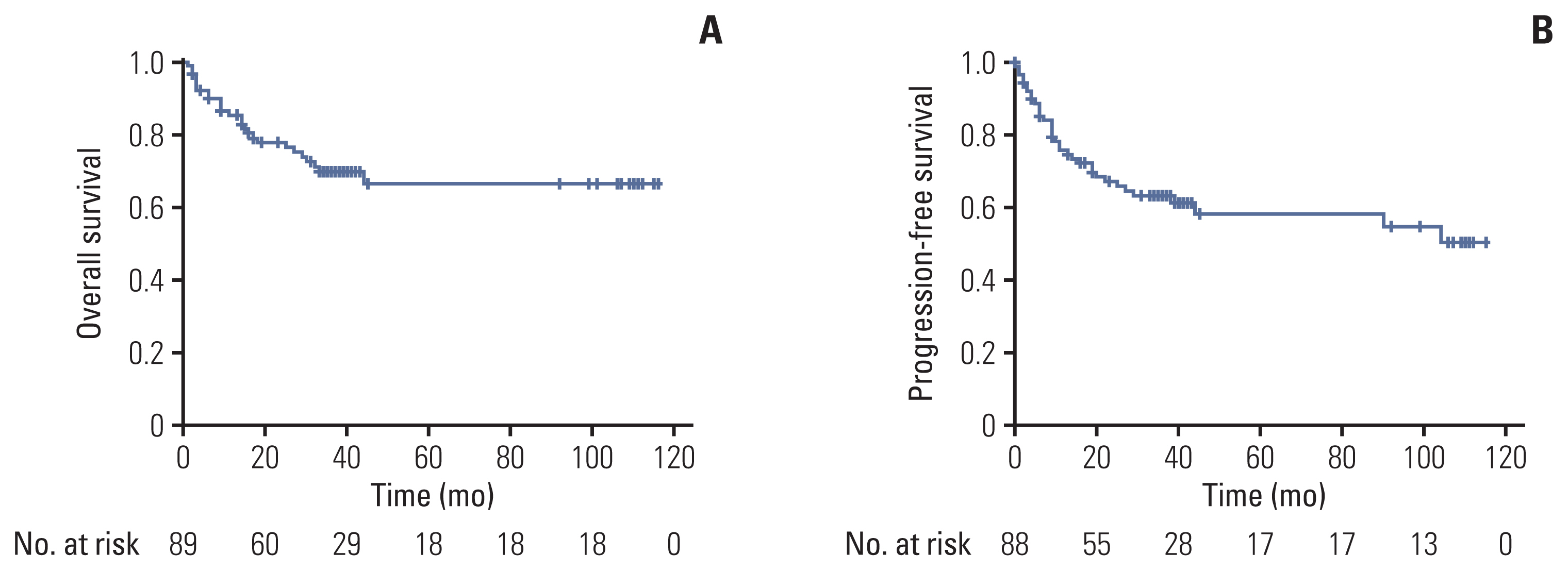
Among the 92 DLBCL patients assessed herein, the response rate after 3 cycles of chemotherapy was 88.0% (38.0% CR+50.0% partial response [PR]). After the completion of 8 cycles of chemotherapy, the overall response rate was observed for 68.4% (58.7% CR+9.8% PR). The 3-year progression-free survival rate was 64.0%, and the 3-year overall survival rate was 70.4%. Febrile neutropenia was one of the most frequent grade 3 adverse events (40.0%) and 5 treatment-related deaths occurred. Compared with the clinical outcomes of patients who received R-CHOP chemotherapy as a historical control, the interim CR rate was higher in male patients with RR-CHOP (20.5% vs. 48.8%, p=0.016).
Conclusion
Rituximab intensification on days 0 to the 1st cycle of the standard 8 cycles R-CHOP-21 for advanced DLBCL yielded favorable response rates after the 3 cycles of chemotherapy and acceptable toxicities, especially for male patients. ClinicalTrials.gov ID: NCT01054781.
Keyword
Figure
Reference
-
References
1. Coiffier B, Thieblemont C, Van Den Neste E, Lepeu G, Plantier I, Castaigne S, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood. 2010; 116:2040–5.2. Pfreundschuh M, Trumper L, Osterborg A, Pettengell R, Trneny M, Imrie K, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006; 7:379–91.3. Pfreundschuh M, Schubert J, Ziepert M, Schmits R, Mohren M, Lengfelder E, et al. Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised control-led trial (RICOVER-60). Lancet Oncol. 2008; 9:105–16.4. Pfreundschuh M, Ho AD, Cavallin-Stahl E, Wolf M, Pettengell R, Vasova I, et al. Prognostic significance of maximum tumour (bulk) diameter in young patients with good-prognosis diffuse large-B-cell lymphoma treated with CHOP-like chemotherapy with or without rituximab: an exploratory analysis of the MabThera International Trial Group (MInT) study. Lancet Oncol. 2008; 9:435–44.5. Yoo KH, Lee H, Suh C. CISL. Lymphoma epidemiology in Korea and the real clinical field including the Consortium for Improving Survival of Lymphoma (CISL) trial. Int J Hematol. 2018; 107:395–404.6. Stiff PJ, Unger JM, Cook JR, Constine LS, Couban S, Stewart DA, et al. Autologous transplantation as consolidation for aggressive non-Hodgkin’s lymphoma. N Engl J Med. 2013; 369:1681–90.7. Muller C, Murawski N, Wiesen MH, Held G, Poeschel V, Zeynalova S, et al. The role of sex and weight on rituximab clearance and serum elimination half-life in elderly patients with DLBCL. Blood. 2012; 119:3276–84.8. Suh C, Park BB, Kim WS; CISL. The Consortium for Improving Survival of Lymphoma (CISL): recent achievements and future perspective. Blood Res. 2017; 52:3–6.9. Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004; 103:275–82.10. Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007; 25:579–86.11. Delarue R, Tilly H, Mounier N, Petrella T, Salles G, Thieblemont C, et al. Dose-dense rituximab-CHOP compared with standard rituximab-CHOP in elderly patients with diffuse large B-cell lymphoma (the LNH03-6B study): a randomised phase 3 trial. Lancet Oncol. 2013; 14:525–33.12. Pfreundschuh M, Kuhnt E, Trumper L, Osterborg A, Trneny M, Shepherd L, et al. CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol. 2011; 12:1013–22.13. Leonard JP, Kolibaba KS, Reeves JA, Tulpule A, Flinn IW, Kolevska T, et al. Randomized phase II study of R-CHOP with or without bortezomib in previously untreated patients with non-germinal center B-cell-like diffuse large B-cell lymphoma. J Clin Oncol. 2017; 35:3538–46.14. Seymour JF, Pfreundschuh M, Trneny M, Sehn LH, Catalano J, Csinady E, et al. R-CHOP with or without bevacizumab in patients with previously untreated diffuse large B-cell lymphoma: final MAIN study outcomes. Haematologica. 2014; 99:1343–9.15. Nowakowski GS, LaPlant B, Macon WR, Reeder CB, Foran JM, Nelson GD, et al. Lenalidomide combined with R-CHOP overcomes negative prognostic impact of non-germinal center B-cell phenotype in newly diagnosed diffuse large B-Cell lymphoma: a phase II study. J Clin Oncol. 2015; 33:251–7.16. Younes A, Sehn LH, Johnson P, Zinzani PL, Hong X, Zhu J, et al. Randomized phase III trial of ibrutinib and rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in non-germinal center B-cell diffuse large B-cell lymphoma. J Clin Oncol. 2019; 37:1285–95.17. Pfreundschuh M, Poeschel V, Zeynalova S, Hanel M, Held G, Schmitz N, et al. Optimization of rituximab for the treatment of diffuse large B-cell lymphoma (II): extended rituximab exposure time in the SMARTE-R-CHOP-14 trial of the german high-grade non-Hodgkin lymphoma study group. J Clin Oncol. 2014; 32:4127–33.18. Murawski N, Pfreundschuh M, Zeynalova S, Poeschel V, Hanel M, Held G, et al. Optimization of rituximab for the treatment of DLBCL (I): dose-dense rituximab in the DENSE-R-CHOP-14 trial of the DSHNHL. Ann Oncol. 2014; 25:1800–6.19. Lugtenburg PJ, de Nully Brown P, van der Holt B, D’Amore FA, Koene HR, de Jongh E, et al. Rituximab-CHOP with early rituximab intensification for diffuse large B-cell lymphoma: a randomized phase III trial of the HOVON and the Nordic Lymphoma Group (HOVON-84). J Clin Oncol. 2020; 38:3377–87.20. Gleeson M, Counsell N, Cunningham D, Lawrie A, Clifton-Hadley L, Hawkes E, et al. Prognostic indices in diffuse large B-cell lymphoma in the rituximab era: an analysis of the UK National Cancer Research Institute R-CHOP 14 versus 21 phase 3 trial. Br J Haematol. 2021; 192:1015–9.21. Li X, Huang H, Xu B, Guo H, Lin Y, Ye S, et al. Dose-dense rituximab-CHOP versus standard rituximab-CHOP in newly diagnosed Chinese patients with diffuse large B-cell lymphoma: a randomized, multicenter, open-label phase 3 trial. Cancer Res Treat. 2019; 51:919–32.22. Cunningham D, Hawkes EA, Jack A, Qian W, Smith P, Mouncey P, et al. Rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone in patients with newly diagnosed diffuse large B-cell non-Hodgkin lymphoma: a phase 3 comparison of dose intensification with 14-day versus 21-day cycles. Lancet. 2013; 381:1817–26.23. Ohmachi K, Kinoshita T, Tobinai K, Ogawa G, Mizutani T, Yamauchi N, et al. A randomized phase 2/3 study of R-CHOP vs CHOP combined with dose-dense rituximab for DLBCL: the JCOG0601 trial. Blood Adv. 2021; 5:984–93.24. Hedstrom G, Peterson S, Berglund M, Jerkeman M, Enblad G; Swedish Lymphoma Study Group. Male gender is an adverse risk factor only in young patients with diffuse large B-cell lymphoma: a Swedish population-based study. Acta Oncol. 2015; 54:924–32.25. Pfreundschuh M, Muller C, Zeynalova S, Kuhnt E, Wiesen MH, Held G, et al. Suboptimal dosing of rituximab in male and female patients with DLBCL. Blood. 2014; 123:640–6.26. Cook MB, McGlynn KA, Devesa SS, Freedman ND, Anderson WF. Sex disparities in cancer mortality and survival. Cancer Epidemiol Biomarkers Prev. 2011; 20:1629–37.27. Micheli A, Ciampichini R, Oberaigner W, Ciccolallo L, de Vries E, Izarzugaza I, et al. The advantage of women in cancer survival: an analysis of EUROCARE-4 data. Eur J Cancer. 2009; 45:1017–27.28. Riihijarvi S, Taskinen M, Jerkeman M, Leppa S. Male gender is an adverse prognostic factor in B-cell lymphoma patients treated with immunochemotherapy. Eur J Haematol. 2011; 86:124–8.29. Pfreundschuh M, Held G, Zeynalova S, Zwick C, Haenel M, Truemper L, et al. Increased rituximab (R) doses and effect on risk of elderly male patients with aggressive CD20+ B-cell lymphomas: results from the SEXIE-R-CHOP-14 trial of the DSHNHL. J Clin Oncol. 2014; 32(15 Suppl):8501.30. Tilly H, Morschhauser F, Sehn LH, Friedberg JW, Trneny M, Sharman JP, et al. Polatuzumab vedotin in previously untreated diffuse large B-cell lymphoma. N Engl J Med. 2022; 386:351–63.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Radioimmunotherapy with 131I-Rituximab in a Patient with Diffuse Large B-Cell Lymphoma Relapsed After Treatment with 90Y-Ibritumomab Tiuxetan
- Rituximab Plus CHOP for the Treatment of Primary Mediastinal Large B Cell Lymphoma in a Pregnant Woman
- Primary diffuse large B-cell lymphoma of the bone marrow in a frail and elderly patient successfully treated with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone
- Relevance of prognostic index with β2-microglobulin for patients with diffuse large B-cell lymphoma in the rituximab era
- A Case of Hepatitis B Virus Reactivation in a HBsAg-Negative and Anti-HBs-Positive Patient with Diffuse Large B-Cell Lymphoma after Rituximab plus CHOP Chemotherapy