Blood Res.
2017 Dec;52(4):276-284. 10.5045/br.2017.52.4.276.
Relevance of prognostic index with β2-microglobulin for patients with diffuse large B-cell lymphoma in the rituximab era
- Affiliations
-
- 1Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. csuh@amc.seoul.kr
- KMID: 2405064
- DOI: http://doi.org/10.5045/br.2017.52.4.276
Abstract
- BACKGROUND
The International Prognostic Index (IPI) has been a useful tool for predicting the prognosis of aggressive non-Hodgkin lymphoma in the last 20 years. Herein, we aimed to develop a new prognostic model for diffuse large B-cell lymphoma (DLBCL) in the rituximab era.
METHODS
Between March 2004 and June 2012, patients with DLBCL treated with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone chemotherapy regimen were identified in the database of the Asan Medical Center (AMC) Lymphoma Registry. The primary and secondary endpoints were a new prognostic index for DLBCL and validation of the National Comprehensive Cancer Network-International Prognostic Index in our cohort, respectively.
RESULTS
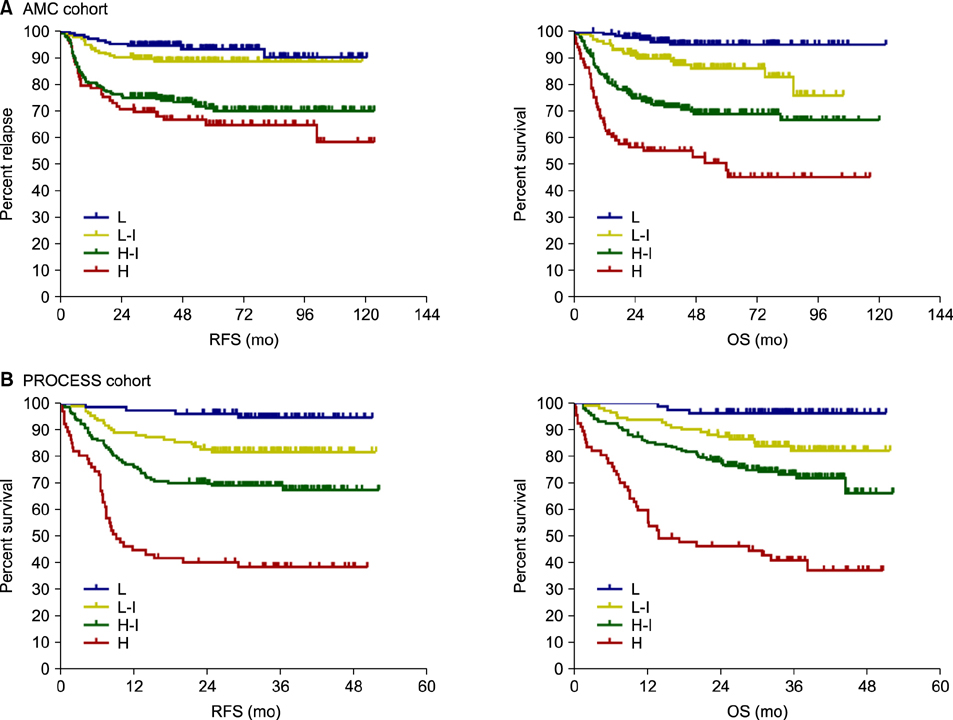
The AMC cohort comprised 621 patients. The median follow-up duration was 43.3 months (range, 6.2-122.5 mo). Univariate analysis revealed that age (≤60 vs. >60 yr), lactate dehydrogenase (LDH; within normal vs. increased), Eastern Cooperative Oncology Group performance status (ECOG PS; 0 or 1 vs. ≥2), advanced stage (Ann Arbor stage I/II vs. III/IV), extra-nodal involvement (≤1 vs. >1), B symptoms (no vs. yes), and beta-2 microglobulin (β2MG, ≤2.5 vs. >2.5) can be used to predict overall survival (OS). In multivariate analysis, only age, LDH, ECOG performance status, and β2MG were significantly associated with OS, and we developed a new prognostic model with these 4 factors. The new prognostic model showed better discriminative power compared with the classic IPI.
CONCLUSION
Our new prognostic index model for DLBCL in the rituximab era has good discriminative power and is convenient to use.
MeSH Terms
-
B-Lymphocytes*
Chungcheongnam-do
Cohort Studies
Cyclophosphamide
Doxorubicin
Drug Therapy
Follow-Up Studies
Humans
L-Lactate Dehydrogenase
Lymphoma
Lymphoma, B-Cell*
Lymphoma, Non-Hodgkin
Multivariate Analysis
Prednisolone
Prognosis
Rituximab*
Vincristine
Cyclophosphamide
Doxorubicin
L-Lactate Dehydrogenase
Prednisolone
Rituximab
Vincristine
Figure
Reference
-
1. Vose JM, Link BK, Grossbard ML, et al. Phase II study of rituximab in combination with chop chemotherapy in patients with previously untreated, aggressive non-Hodgkin's lymphoma. J Clin Oncol. 2001; 19:389–397.
Article2. Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002; 346:235–242.
Article3. Feugier P, Van Hoof A, Sebban C, et al. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d'Etude des Lymphomes de l'Adulte. J Clin Oncol. 2005; 23:4117–4126.
Article4. Pfreundschuh M, Trümper L, Osterborg A, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006; 7:379–391.5. Pfreundschuh M, Schubert J, Ziepert M, et al. Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60). Lancet Oncol. 2008; 9:105–116.6. International Non-Hodgkin's Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med. 1993; 329:987–994.7. Ziepert M, Hasenclever D, Kuhnt E, et al. Standard International prognostic index remains a valid predictor of outcome for patients with aggressive CD20+ B-cell lymphoma in the rituximab era. J Clin Oncol. 2010; 28:2373–2380.
Article8. Sehn LH, Berry B, Chhanabhai M, et al. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood. 2007; 109:1857–1861.
Article9. Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000; 403:503–511.
Article10. Green TM, Young KH, Visco C, et al. Immunohistochemical double-hit score is a strong predictor of outcome in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012; 30:3460–3467.
Article11. Zhou Z, Sehn LH, Rademaker AW, et al. An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood. 2014; 123:837–842.
Article12. Shi C, Zhu Y, Su Y, Chung LW, Cheng T. Beta2-microglobulin: emerging as a promising cancer therapeutic target. Drug Discov Today. 2009; 14:25–30.13. Yoo C, Yoon DH, Yoon S, et al. Prognostic impact of β2-microglobulin in patients with non-gastric mucosa-associated lymphoid tissue lymphoma. Leuk Lymphoma. 2015; 56:688–693.
Article14. Yoo C, Yoon DH, Jo JC, et al. Prognostic impact of beta-2 microglobulin in patients with extranodal natural killer/T cell lymphoma. Ann Hematol. 2014; 93:995–1000.
Article15. Yoo C, Yoon DH, Suh C. Serum beta-2 microglobulin in malignant lymphomas: an old but powerful prognostic factor. Blood Res. 2014; 49:148–153.
Article16. Duletić-Nacinović A, Stifter S, Marijić B, et al. Serum IL-6, IL-8, IL-10 and beta2-microglobulin in association with International Prognostic Index in diffuse large B cell lymphoma. Tumori. 2008; 94:511–517.
Article17. Melchardt T, Troppan K, Weiss L, et al. A modified scoring of the NCCN-IPI is more accurate in the elderly and is improved by albumin and β2 -microglobulin. Br J Haematol. 2015; 168:239–245.
Article18. Öztürk E, Özbalak M, Berk S, et al. Comparison of International Prognostic Index and NCCN-IPI in 324 patients with de novo diffuse large B-cell lymphoma: a multi-center retrospective analysis. Leuk Lymphoma. 2016; 57:1211–1214.
Article19. Mian M, Marcheselli L, Rossi A, et al. A diachronic-comparative analysis for the identification of the most powerful prognostic index for localized diffuse large B-cell lymphoma. Ann Oncol. 2014; 25:2398–2404.
Article20. Nakajima Y, Tomita N, Itabashi M, et al. Analysis of outcomes in patients with supra-diaphragmatic vs infra-diaphragmatic diffuse large B cell lymphoma treated with R-CHOP therapy. Leuk Res. 2015; 39:198–203.
Article21. Kuo SH, Yeh KH, Chen LT, et al. Helicobacter pylori-related diffuse large B-cell lymphoma of the stomach: a distinct entity with lower aggressiveness and higher chemosensitivity. Blood Cancer J. 2014; 4:e220.
Article22. Solal-Céligny P, Roy P, Colombat P, et al. Follicular lymphoma international prognostic index. Blood. 2004; 104:1258–1265.
Article23. López-Guillermo A, Colomo L, Jiménez M, et al. Diffuse large B-cell lymphoma: clinical and biological characterization and outcome according to the nodal or extranodal primary origin. J Clin Oncol. 2005; 23:2797–2804.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A new era of B cell lymphoma treatment by using monoclonal antibody: Rituximab (anti-CD20 antibody)
- Is there a relationship between the infusion-related reaction and effect of rituximab in the treatment of patients with diffuse large B-cell lymphoma?
- Serum beta-2 microglobulin in malignant lymphomas: an old but powerful prognostic factor
- Treatment of Diffuse Large B Cell Lymphoma
- Primary Diffuse Large B Cell Lymphoma of the Bladder