Int J Stem Cells.
2023 Aug;16(3):281-292. 10.15283/ijsc22158.
Effect of Xenogeneic Substances on the Glycan Profiles and Electrophysiological Properties of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes
- Affiliations
-
- 1Advanced Bioconvergence Product Research Division, National Institute of Food and Drug Safety Evaluation, Ministry of Food and Drug Safety, Cheongju, Korea
- 2Department of Predictive Toxicology, Korea Institute of Toxicology, Daejeon, Korea
- KMID: 2545228
- DOI: http://doi.org/10.15283/ijsc22158
Abstract
- Background and Objectives
Human induced pluripotent stem cell (hiPSC)-derived cardiomyocyte (CM) hold great promise as a cellular source of CM for cardiac function restoration in ischemic heart disease. However, the use of animal-derived xenogeneic substances during the biomanufacturing of hiPSC-CM can induce inadvertent immune responses or chronic inflammation, followed by tumorigenicity. In this study, we aimed to reveal the effects of xenogeneic substances on the functional properties and potential immunogenicity of hiPSC-CM during differentiation, demonstrating the quality and safety of hiPSC-based cell therapy.
Methods and Results
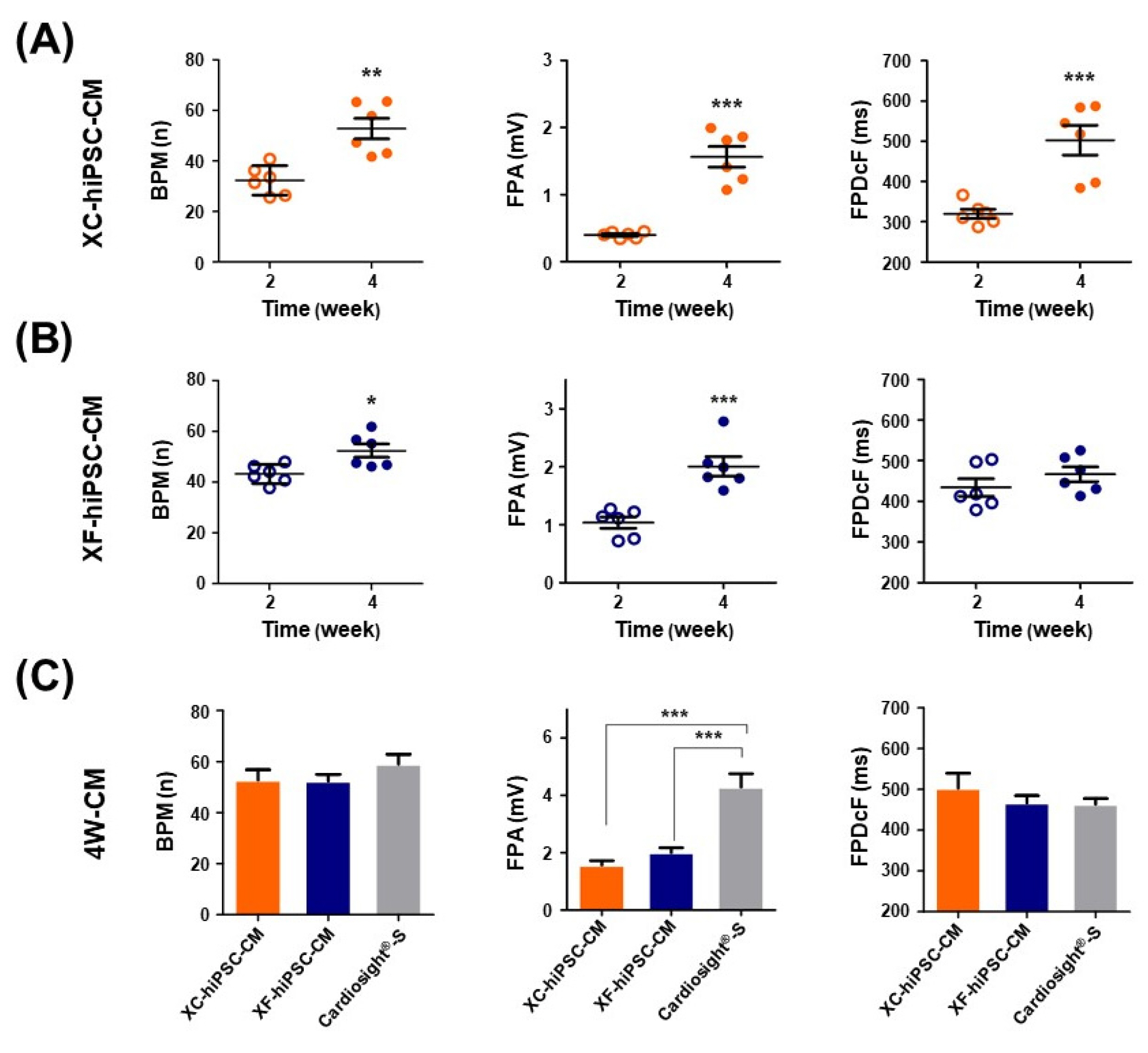
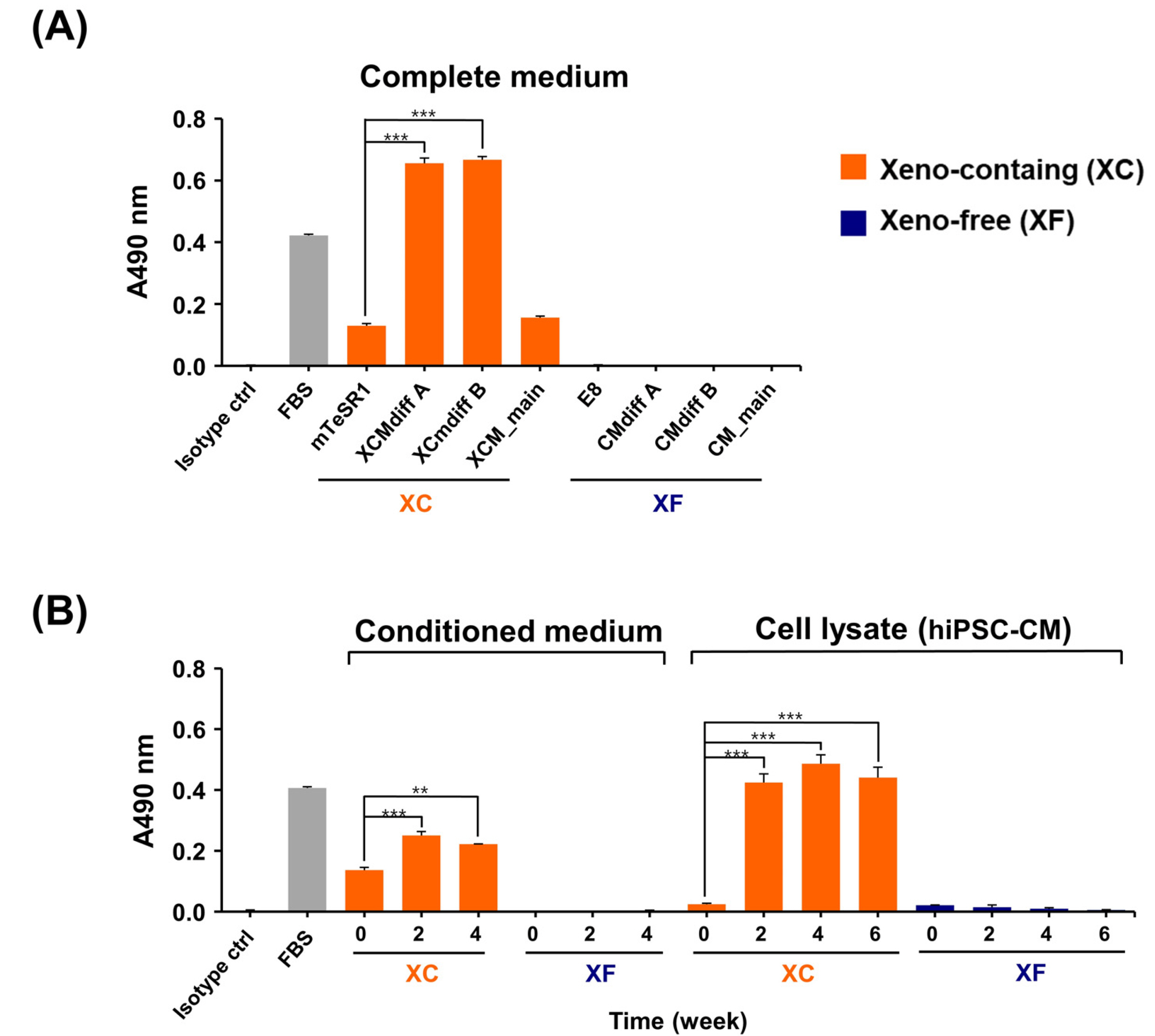
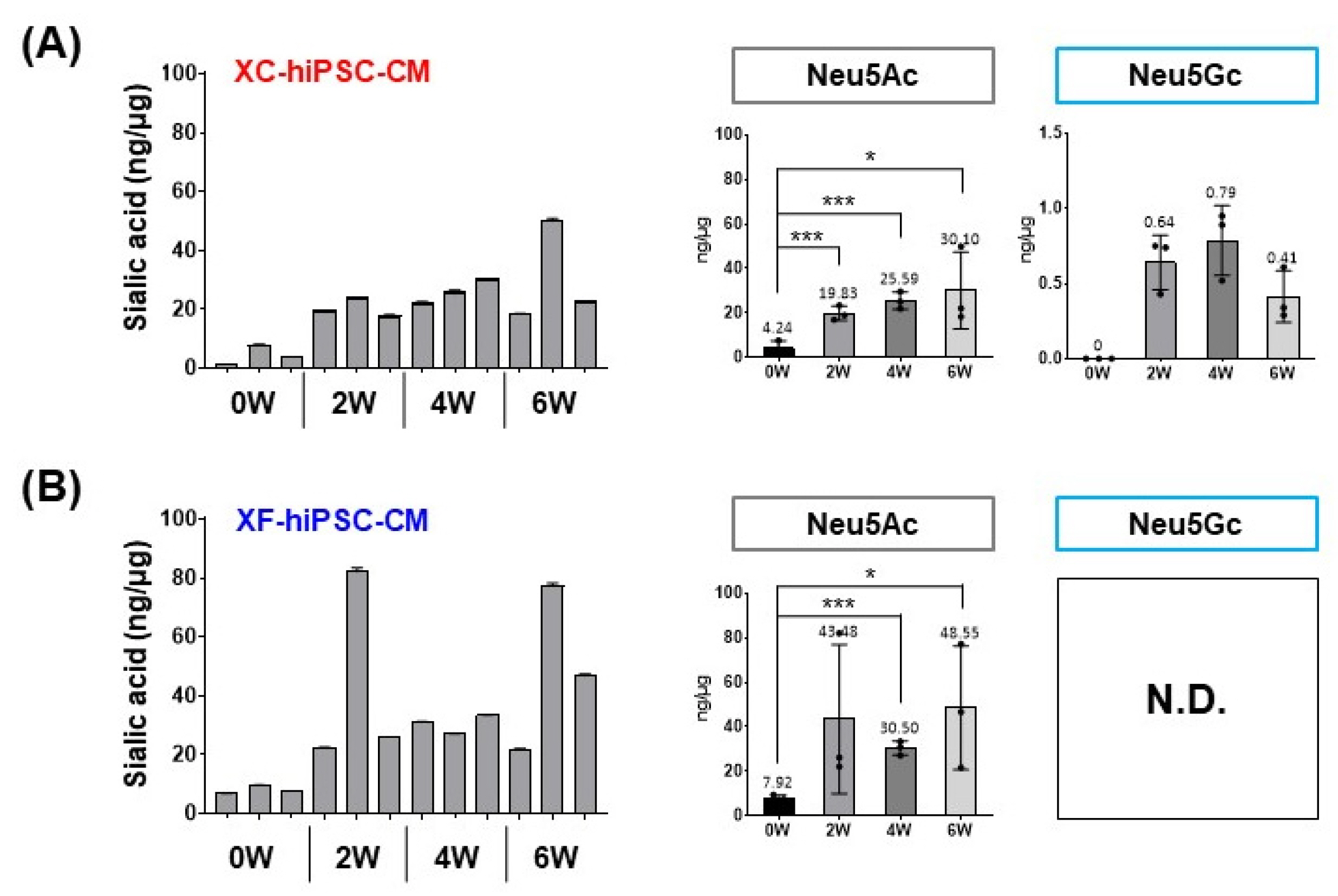
We successfully generated hiPSC-CM in the presence and absence of xenogeneic substances (xeno-containing (XC) and xeno-free (XF) conditions, respectively), and compared their characteristics, including the contractile functions and glycan profiles. Compared to XC-hiPSC-CM, XF-hiPSC-CM showed early onset of myocyte contractile beating and maturation, with a high expression of cardiac lineage-specific genes (ACTC1, TNNT2, and RYR2) by using MEA and RT-qPCR. We quantified N-glycolylneuraminic acid (Neu5Gc), a xenogeneic sialic acid, in hiPSC-CM using an indirect enzyme-linked immunosorbent assay and liquid chromatography-multiple reaction monitoring-mass spectrometry. Neu5Gc was incorporated into the glycans of hiPSC-CM during xeno-containing differentiation, whereas it was barely detected in XF-hiPSC-CM.
Conclusions
To the best of our knowledge, this is the first study to show that the electrophysiological function and glycan profiles of hiPSC-CM can be affected by the presence of xenogeneic substances during their differentiation and maturation. To ensure quality control and safety in hiPSC-based cell therapy, xenogeneic substances should be excluded from the biomanufacturing process.
Keyword
Figure
Reference
-
References
1. Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, Boehme AK, Buxton AE, Carson AP, Commodore-Mensah Y, Elkind MSV, Evenson KR, Eze-Nliam C, Ferguson JF, Generoso G, Ho JE, Kalani R, Khan SS, Kissela BM, Knutson KL, Levine DA, Lewis TT, Liu J, Loop MS, Ma J, Mussolino ME, Navaneethan SD, Perak AM, Poudel R, Rezk-Hanna M, Roth GA, Schroeder EB, Shah SH, Thacker EL, VanWagner LB, Virani SS, Voecks JH, Wang NY, Yaffe K, Martin SS. 2022; Heart Disease and Stroke Statistics-2022 update: a report from the American Heart Association. Circulation. 145:e153–e639. DOI: 10.1161/CIR.0000000000001052. PMID: 35078371.
Article2. Liu YW, Chen B, Yang X, Fugate JA, Kalucki FA, Futaku-chi-Tsuchida A, Couture L, Vogel KW, Astley CA, Balde-ssari A, Ogle J, Don CW, Steinberg ZL, Seslar SP, Tuck SA, Tsuchida H, Naumova AV, Dupras SK, Lyu MS, Lee J, Hailey DW, Reinecke H, Pabon L, Fryer BH, MacLellan WR, Thies RS, Murry CE. 2018; Human embryonic stem cell-derived cardiomyocytes restore function in infarcted hearts of non-human primates. Nat Biotechnol. 36:597–605. DOI: 10.1038/nbt.4162. PMID: 29969440. PMCID: PMC6329375.
Article3. Karbassi E, Fenix A, Marchiano S, Muraoka N, Nakamura K, Yang X, Murry CE. 2020; Cardiomyocyte maturation: advances in knowledge and implications for regenerative medicine. Nat Rev Cardiol. 17:341–359. DOI: 10.1038/s41569-019-0331-x. PMID: 32015528. PMCID: PMC7239749.
Article4. Chong JJ, Yang X, Don CW, Minami E, Liu YW, Weyers JJ, Mahoney WM, Van Biber B, Cook SM, Palpant NJ, Gantz JA, Fugate JA, Muskheli V, Gough GM, Vogel KW, Astley CA, Hotchkiss CE, Baldessari A, Pabon L, Reinecke H, Gill EA, Nelson V, Kiem HP, Laflamme MA, Murry CE. 2014; Human embryonic-stem-cell-derived cardiomyocytes rege-nerate non-human primate hearts. Nature. 510:273–277. DOI: 10.1038/nature13233. PMID: 24776797. PMCID: PMC4154594.
Article5. Lian X, Hsiao C, Wilson G, Zhu K, Hazeltine LB, Azarin SM, Raval KK, Zhang J, Kamp TJ, Palecek SP. 2012; Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc Natl Acad Sci U S A. 109:E1848–E1857. DOI: 10.1073/pnas.1200250109. PMID: 22645348. PMCID: PMC3390875.
Article6. Martin MJ, Muotri A, Gage F, Varki A. 2005; Human embryonic stem cells express an immunogenic nonhuman sialic acid. Nat Med. 11:228–232. DOI: 10.1038/nm1181. PMID: 15685172.
Article7. Herberts CA, Kwa MS, Hermsen HP. 2011; Risk factors in the development of stem cell therapy. J Transl Med. 9:29. DOI: 10.1186/1479-5876-9-29. PMID: 21418664. PMCID: PMC3070641.
Article8. Lin Y, Zou J. 2020; Differentiation of cardiomyocytes from human pluripotent stem cells in fully chemically defined conditions. STAR Protoc. 1:100015. DOI: 10.1016/j.xpro.2020.100015. PMID: 32734277. PMCID: PMC7392178.
Article9. Ashok P, Parikh A, Du C, Tzanakakis ES. 2020; Xenogeneic-free system for biomanufacturing of cardiomyocyte progeny from human pluripotent stem cells. Front Bioeng Biotech-nol. 8:571425. DOI: 10.3389/fbioe.2020.571425. PMID: 33195131. PMCID: PMC7644809.
Article10. Chou HH, Takematsu H, Diaz S, Iber J, Nickerson E, Wright KL, Muchmore EA, Nelson DL, Warren ST, Varki A. 1998; A mutation in human CMP-sialic acid hydroxylase occurred after the Homo-Pan divergence. Proc Natl Acad Sci U S A. 95:11751–11756. DOI: 10.1073/pnas.95.20.11751. PMID: 9751737. PMCID: PMC21712.
Article11. Irie A, Koyama S, Kozutsumi Y, Kawasaki T, Suzuki A. 1998; The molecular basis for the absence of N-glycolylneuraminic acid in humans. J Biol Chem. 273:15866–15871. DOI: 10.1074/jbc.273.25.15866. PMID: 9624188.
Article12. Varki A, Schnaar RL, Schauer R. Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, Darvill AG, Kinoshita T, Packer NH, Prestegard JH, Schnaar RL, Seeberger PH, editors. 2015. Sialic acids and other nonulosonic acids. Essentials of Glycobiology. 3rd ed. Cold Spring Harbor Laboratory Press;Cold Spring Harbor: p. 179–195.13. Tangvoranuntakul P, Gagneux P, Diaz S, Bardor M, Varki N, Varki A, Muchmore E. 2003; Human uptake and incorpo-ration of an immunogenic nonhuman dietary sialic acid. Proc Natl Acad Sci U S A. 100:12045–12050. DOI: 10.1073/pnas.2131556100. PMID: 14523234. PMCID: PMC218710.
Article14. Hedlund M, Padler-Karavani V, Varki NM, Varki A. 2008; Evidence for a human-specific mechanism for diet and antibody-mediated inflammation in carcinoma progression. Proc Natl Acad Sci U S A. 105:18936–18941. DOI: 10.1073/pnas.0803943105. PMID: 19017806. PMCID: PMC2596253.
Article15. Bardor M, Nguyen DH, Diaz S, Varki A. 2005; Mechanism of uptake and incorporation of the non-human sialic acid N-glycolylneuraminic acid into human cells. J Biol Chem. 280:4228–4237. DOI: 10.1074/jbc.M412040200. PMID: 15557321.
Article16. Padler-Karavani V, Hurtado-Ziola N, Pu M, Yu H, Huang S, Muthana S, Chokhawala HA, Cao H, Secrest P, Friedmann-Morvinski D, Singer O, Ghaderi D, Verma IM, Liu YT, Messer K, Chen X, Varki A, Schwab R. 2011; Human xeno-autoantibodies against a non-human sialic acid serve as novel serum biomarkers and immunotherapeutics in cancer. Cancer Res. 71:3352–3363. DOI: 10.1158/0008-5472.CAN-10-4102. PMID: 21505105. PMCID: PMC3085609.
Article17. Samraj AN, Pearce OM, Läubli H, Crittenden AN, Bergfeld AK, Banda K, Gregg CJ, Bingman AE, Secrest P, Diaz SL, Varki NM, Varki A. 2015; A red meat-derived glycan promotes inflammation and cancer progression. Proc Natl Acad Sci U S A. 112:542–547. DOI: 10.1073/pnas.1417508112. PMID: 25548184. PMCID: PMC4299224.
Article18. Bashir S, Fezeu LK, Leviatan Ben-Arye S, Yehuda S, Reuven EM, Szabo de Edelenyi F, Fellah-Hebia I, Le Tourneau T, Imbert-Marcille BM, Drouet EB, Touvier M, Roussel JC, Yu H, Chen X, Hercberg S, Cozzi E, Soulillou JP, Galan P, Padler-Karavani V. 2020; Association between Neu5Gc carbohydrate and serum antibodies against it provides the molecular link to cancer: French NutriNet-Santé study. BMC Med. 18:262. DOI: 10.1186/s12916-020-01721-8. PMID: 32962714. PMCID: PMC7510162.
Article19. Lian X, Zhang J, Azarin SM, Zhu K, Hazeltine LB, Bao X, Hsiao C, Kamp TJ, Palecek SP. 2013; Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions. Nat Protoc. 8:162–175. DOI: 10.1038/nprot.2012.150. PMID: 23257984. PMCID: PMC3612968.
Article20. Lee SJ, Kim HA, Kim SJ, Lee HA. 2021; Improving generation of cardiac organoids from human pluripotent stem cells using the aurora kinase inhibitor ZM447439. Biomedicines. 9:1952. DOI: 10.3390/biomedicines9121952. PMID: 34944767. PMCID: PMC8698385.21. Seo N, Ko J, Lee D, Jeong H, Oh MJ, Kim U, Lee DH, Kim J, Choi YJ, An HJ. 2021; In-depth characterization of non-human sialic acid (Neu5Gc) in human serum using label-free ZIC-HILIC/MRM-MS. Anal Bioanal Chem. 413:5227–5237. DOI: 10.1007/s00216-021-03495-1. PMID: 34235565.
Article22. Hua S, Saunders M, Dimapasoc LM, Jeong SH, Kim BJ, Kim S, So M, Lee KS, Kim JH, Lam KS, Lebrilla CB, An HJ. 2014; Differentiation of cancer cell origin and molecular subtype by plasma membrane N-glycan profiling. J Proteome Res. 13:961–968. DOI: 10.1021/pr400987f. PMID: 24303873. PMCID: PMC3946297.
Article23. Oh MJ, Hua S, Kim BJ, Jeong HN, Jeong SH, Grimm R, Yoo JS, An HJ. 2013; Analytical platform for glycomic characterization of recombinant erythropoietin biotherapeutics and biosimilars by MS. Bioanalysis. 5:545–559. DOI: 10.4155/bio.12.327. PMID: 23425271.
Article24. Mummery CL, Zhang J, Ng ES, Elliott DA, Elefanty AG, Kamp TJ. 2012; Differentiation of human embryonic stem cells and induced pluripotent stem cells to cardiomyocytes: a methods overview. Circ Res. 111:344–358. DOI: 10.1161/CIRCRESAHA.110.227512. PMID: 22821908. PMCID: PMC3578601.
Article25. Cahan P, Li H, Morris SA, Lummertz da Rocha E, Daley GQ, Collins JJ. 2014; CellNet: network biology applied to stem cell engineering. Cell. 158:903–915. DOI: 10.1016/j.cell.2014.07.020. PMID: 25126793. PMCID: PMC4233680.
Article26. Jung G, Fajardo G, Ribeiro AJ, Kooiker KB, Coronado M, Zhao M, Hu DQ, Reddy S, Kodo K, Sriram K, Insel PA, Wu JC, Pruitt BL, Bernstein D. 2016; Time-dependent evolution of functional vs. remodeling signaling in induced pluripotent stem cell-derived cardiomyocytes and induced maturation with biomechanical stimulation. FASEB J. 30:1464–1479. DOI: 10.1096/fj.15-280982. PMID: 26675706. PMCID: PMC4799510.
Article27. Ojala M, Rajala K, Pekkanen-Mattila M, Miettinen M, Huhtala H, Aalto-Setälä K. 2012; Culture conditions affect cardiac differentiation potential of human pluripotent stem cells. PLoS One. 7:e48659. DOI: 10.1371/journal.pone.0048659. PMID: 23119085. PMCID: PMC3485380.
Article28. Feaster TK, Cadar AG, Wang L, Williams CH, Chun YW, Hempel JE, Bloodworth N, Merryman WD, Lim CC, Wu JC, Knollmann BC, Hong CC. 2015; Matrigel mattress: a method for the generation of single contracting human-induced pluripotent stem cell-derived cardiomyocytes. Circ Res. 117:995–1000. DOI: 10.1161/CIRCRESAHA.115.307580. PMID: 26429802. PMCID: PMC4670592.29. Lim JJ, Kim HJ, Rhie BH, Lee MR, Choi MJ, Hong SH, Kim KS. 2019; Maintenance of hPSCs under Xeno-free and che-mically defined culture conditions. Int J Stem Cells. 12:484–496. DOI: 10.15283/ijsc19090. PMID: 31658510. PMCID: PMC6881038.30. Hua Y, Yoshimochi K, Li J, Takekita K, Shimotsuma M, Li L, Qu X, Zhang J, Sawa Y, Liu L, Miyagawa S. 2022; Development and evaluation of a novel xeno-free culture medium for human-induced pluripotent stem cells. Stem Cell Res Ther. 13:223. DOI: 10.1186/s13287-022-02879-z. PMID: 35658933. PMCID: PMC9166585.
Article31. Tateishi K, Ando W, Higuchi C, Hart DA, Hashimoto J, Nakata K, Yoshikawa H, Nakamura N. 2008; Comparison of human serum with fetal bovine serum for expansion and differentiation of human synovial MSC: potential feasibility for clinical applications. Cell Transplant. 17:549–557. DOI: 10.3727/096368908785096024. PMID: 18714674.32. Sung TC, Liu CH, Huang WL, Lee YC, Kumar SS, Chang Y, Ling QD, Hsu ST, Higuchi A. 2019; Efficient differentiation of human ES and iPS cells into cardiomyocytes on biomaterials under xeno-free conditions. Biomater Sci. 7:5467–5481. DOI: 10.1039/C9BM00817A. PMID: 31656967.33. Villa-Diaz LG, Ross AM, Lahann J, Krebsbach PH. 2013; Concise review: the evolution of human pluripotent stem cell culture: from feeder cells to synthetic coatings. Stem Cells. 31:1–7. DOI: 10.1002/stem.1260. PMID: 23081828. PMCID: PMC3537180.
Article34. Henderson CA, Gomez CG, Novak SM, Mi-Mi L, Gregorio CC. 2017; Overview of the muscle cytoskeleton. Compr Physiol. 7:891–944. DOI: 10.1002/cphy.c160033. PMID: 28640448. PMCID: PMC5890934.
Article35. Bosman A, Sartiani L, Spinelli V, Del Lungo M, Stillitano F, Nosi D, Mugelli A, Cerbai E, Jaconi M. 2013; Molecular and functional evidence of HCN4 and caveolin-3 interaction during cardiomyocyte differentiation from human embryonic stem cells. Stem Cells Dev. 22:1717–1727. DOI: 10.1089/scd.2012.0247. PMID: 23311301. PMCID: PMC3657289.
Article36. Medeiros-Domingo A, Bhuiyan ZA, Tester DJ, Hofman N, Bikker H, van Tintelen JP, Mannens MM, Wilde AA, Ackerman MJ. 2009; The RYR2-encoded ryanodine receptor/calcium release channel in patients diagnosed previously with either catecholaminergic polymorphic ventricular tachycardia or genotype negative, exercise-induced long QT syndrome: a comprehensive open reading frame mutational analysis. J Am Coll Cardiol. 54:2065–2074. DOI: 10.1016/j.jacc.2009.08.022. PMID: 19926015. PMCID: PMC2880864.
Article37. Yang HT, Tweedie D, Wang S, Guia A, Vinogradova T, Bogdanov K, Allen PD, Stern MD, Lakatta EG, Boheler KR. 2002; The ryanodine receptor modulates the spontaneous beating rate of cardiomyocytes during development. Proc Natl Acad Sci U S A. 99:9225–9230. DOI: 10.1073/pnas.142651999. PMID: 12089338. PMCID: PMC123122.
Article38. Galili U. 1999; Evolution of alpha 1,3galactosyltransferase and of the alpha-Gal epitope. Subcell Biochem. 32:1–23. DOI: 10.1007/978-1-4615-4771-6_1. PMID: 10391989.39. Chung CH, Mirakhur B, Chan E, Le QT, Berlin J, Morse M, Murphy BA, Satinover SM, Hosen J, Mauro D, Slebos RJ, Zhou Q, Gold D, Hatley T, Hicklin DJ, Platts-Mills TA. 2008; Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med. 358:1109–1117. DOI: 10.1056/NEJMoa074943. PMID: 18337601. PMCID: PMC2361129.
Article40. Swiontek K, Morisset M, Codreanu-Morel F, Fischer J, Mehlich J, Darsow U, Petitpain N, Biedermann T, Ollert M, Eberlein B, Hilger C. 2019; Drugs of porcine origin- a risk for patients with α-gal syndrome? J Allergy Clin Immunol Pract. 7:1687–1690.e3. DOI: 10.1016/j.jaip.2018.12.005. PMID: 30557715.41. Chinuki Y, Morita E. 2019; Alpha-Gal-containing biologics and anaphylaxis. Allergol Int. 68:296–300. DOI: 10.1016/j.alit.2019.04.001. PMID: 31053502.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cardiac Regeneration with Human Pluripotent Stem Cell-Derived Cardiomyocytes
- Is Stem Cell-Based Therapy Going on or Out for Cardiac Disease?
- Stem Cell Properties of Therapeutic Potential
- Maturation of Stem Cell-Derived Cardiomyocytes: Foe in Translation Medicine
- Neuronal Differentiation of a Human Induced Pluripotent Stem Cell Line (FS-1) Derived from Newborn Foreskin Fibroblasts