Cancer Res Treat.
2023 Jul;55(3):766-777. 10.4143/crt.2022.987.
Eflapegrastim versus Pegfilgrastim for Chemotherapy-Induced Neutropenia in Korean and Asian Patients with Early Breast Cancer: Results from the Two Phase III ADVANCE and RECOVER Studies
- Affiliations
-
- 1Hematology and Oncology, Department of Internal Medicine, CHA Bundang Medical Center, CHA University, Seongnam, Korea
- 2Department of Surgery, CHA Bundang Medical Center, CHA University, Seongnam, Korea
- 3Center for Breast Cancer, National Cancer Center, Goyang, Korea
- 4Department of Internal Medicine, Inha University College of Medicine, Incheon, Korea
- 5Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 6Division of Medical Oncology, Department of Internal Medicine, Korea University Anam Hospital, Korea University College of Medicine, Seoul, Korea
- 7Division of Medical Oncology, Department of Internal Medicine, Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, Korea
- 8Department of Hemato-Oncology, Wonju Severance Christian Hospital, Yonsei University Wonju College of Medicine, Wonju, Korea
- 9Clinical Research and Development, Hanmi Pharmaceutical Co., Ltd., Seoul, Korea
- 10Department of Internal Medicine, Seoul National University Hospital, Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea
- KMID: 2544160
- DOI: http://doi.org/10.4143/crt.2022.987
Abstract
- Purpose
We investigated the consistent efficacy and safety of eflapegrastim, a novel long-acting granulocyte-colony stimulating factor (G-CSF), in Koreans and Asians compared with the pooled population of two global phase 3 trials.
Materials and Methods
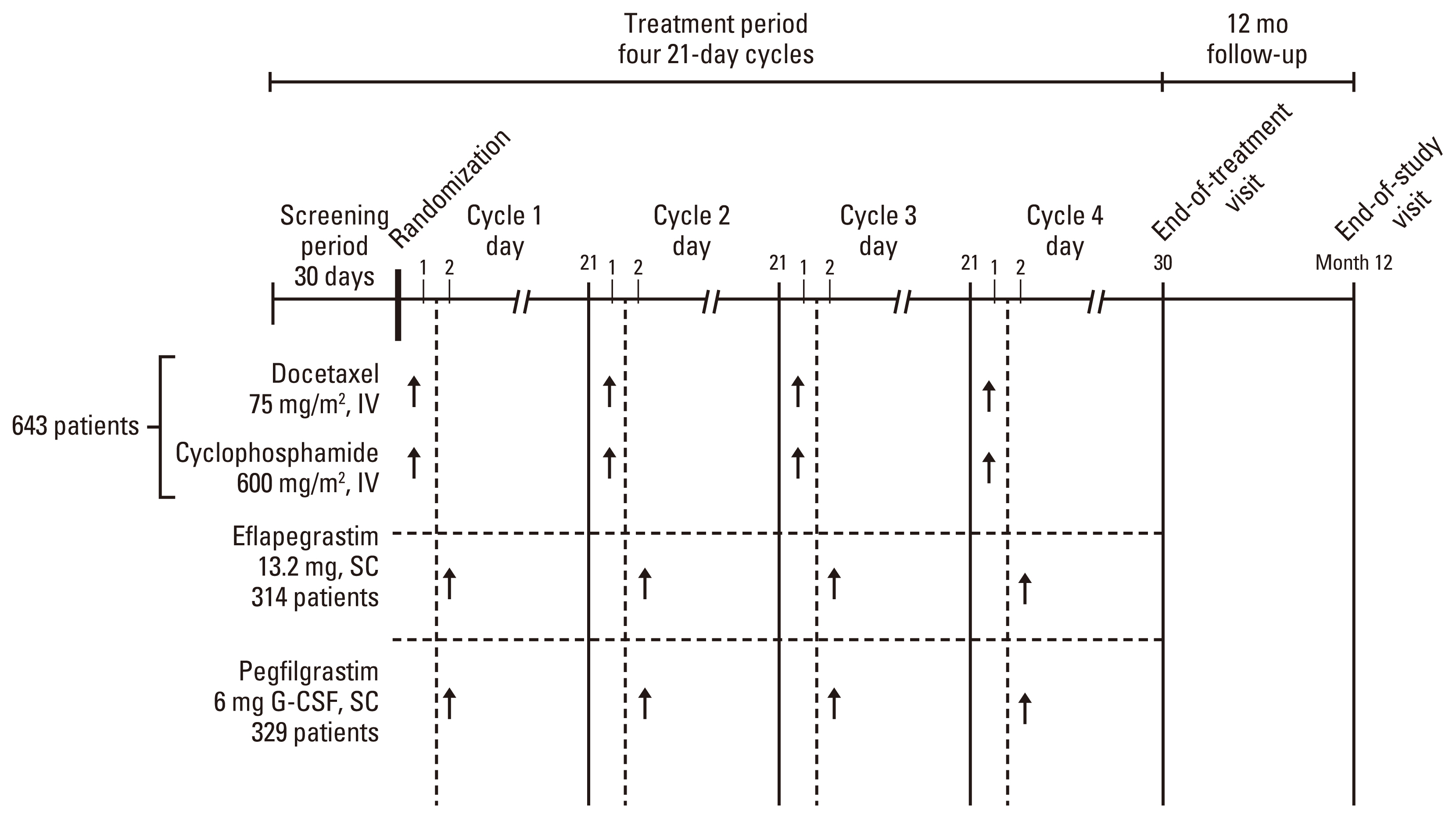
Two phase 3 trials (ADVANCE and RECOVER) evaluated the efficacy and safety of fixed-dose eflapegrastim (13.2 mg/0.6 mL [3.6 mg G-CSF equivalent]) compared to pegfilgrastim (6 mg based on G-CSF) in breast cancer patients who received neoadjuvant or adjuvant docetaxel/cyclophosphamide. The primary objective was to demonstrate non-inferiority of eflapegrastim compared to pegfilgrastim in mean duration of severe neutropenia (DSN) in cycle 1, in Korean and Asian subpopulations.
Results
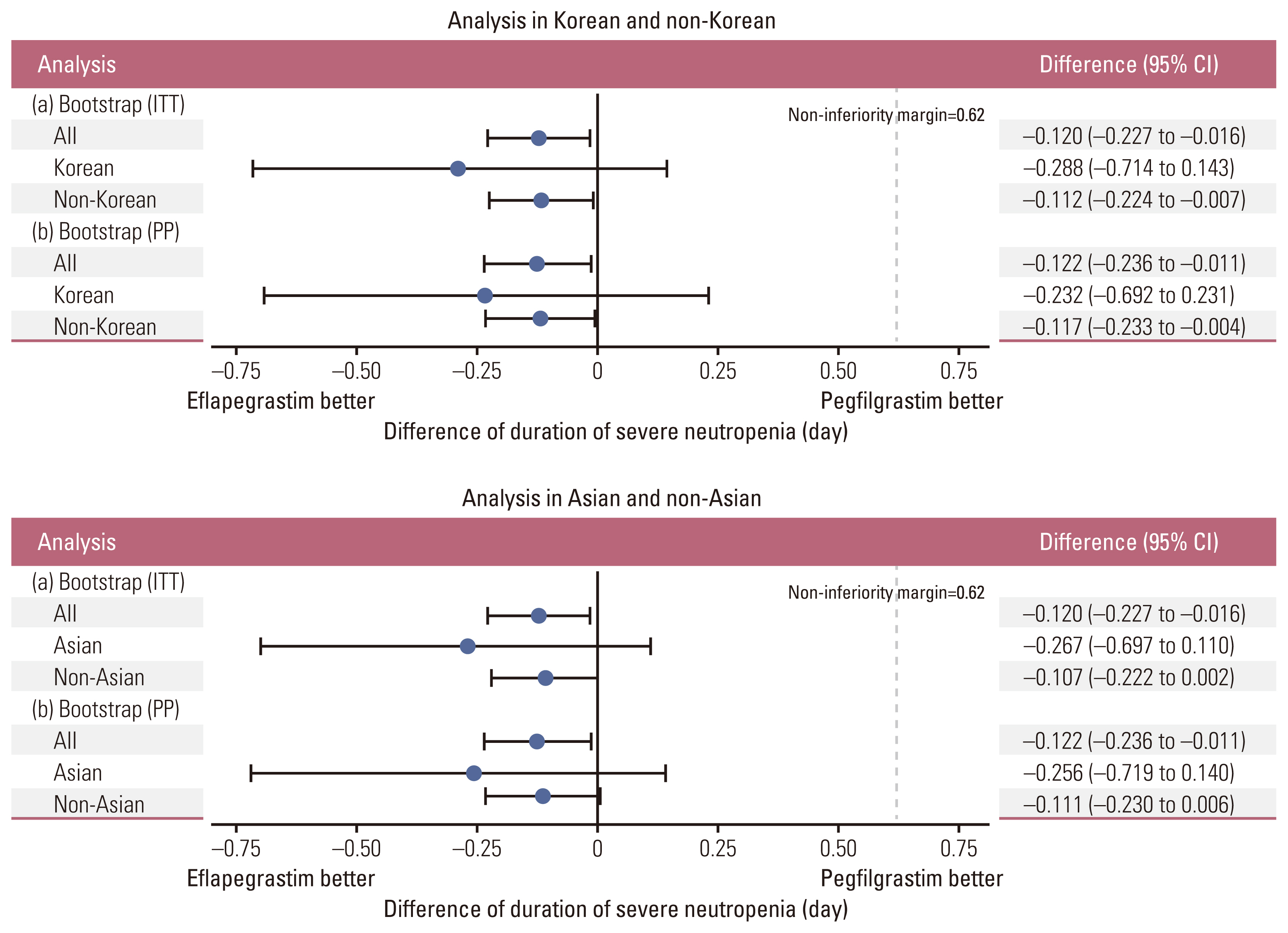
Among a total of 643 patients randomized to eflapegrastim (n=314) or pegfilgrastim (n=329), 54 Asians (29 to eflapegrastim and 25 to pegfilgrastim) including 28 Koreans (14 to both eflapegrastim and pegfilgrastim) were enrolled. The primary endpoint, DSN in cycle 1 in the eflapegrastim arm was non-inferior to the pegfilgrastim arm in Koreans and Asians. The DSN difference between the eflapegrastim and pegfilgrastim arms was consistent across populations: –0.120 days (95% confidence interval [CI], –0.227 to –0.016), –0.288 (95% CI, –0.714 to 0.143), and –0.267 (95% CI, –0.697 to 0.110) for pooled population, Koreans and Asians, respectively. There were few treatment-related adverse events that caused discontinuation of eflapegrastim (1.9%) or pegfilgrastim (1.5%) in total and no notable trends or differences across patient populations.
Conclusion
This study may suggest that eflapegrastim showed non-inferior efficacy and similar safety compared to pegfilgrastim in Koreans and Asians, consistently with those of pooled population.
Keyword
Figure
Reference
-
References
1. Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends: an update. Cancer Epidemiol Biomarkers Prev. 2016; 25:16–27.2. National Comprehensive Cancer Network. Breast cancer, version 2 [Internet]. Plymouth Meeting, PA: National Comprehensive Cancer Network;2017. [cited 2023 Jan 16]. Available from: http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.3. Aapro MS, Bohlius J, Cameron DA, Dal Lago L, Donnelly JP, Kearney N, et al. 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer. 2011; 47:8–32.
Article4. Kuderer NM, Dale DC, Crawford J, Lyman GH. Impact of primary prophylaxis with granulocyte colony-stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: a systematic review. J Clin Oncol. 2007; 25:3158–67.5. Cobb PW, Moon YW, Mezei K, Lang I, Bhat G, Chawla S, et al. A comparison of eflapegrastim to pegfilgrastim in the management of chemotherapy-induced neutropenia in patients with early-stage breast cancer undergoing cytotoxic chemotherapy (RECOVER): p phase 3 study. Cancer Med. 2020; 9:6234–43.
Article6. Schwartzberg LS, Bhat G, Peguero J, Agajanian R, Bharadwaj JS, Restrepo A, et al. Eflapegrastim, a long-acting granulocyte-colony stimulating factor for the management of chemotherapy-induced neutropenia: results of a phase III trial. Oncologist. 2020; 25:e1233–41.
Article7. Barrett JA, Greene D, Lakshmikanthan S, Kolli P, Chawla S, Lebel F. Justification for a fixed dose of eflapegrastim, a long-acting G-CSF, in patients receiving docetaxel-cyclophosphamide chemotherapy. J Clin Pharmacol. 2021; 61:204–10.
Article8. Arvedson T, O’Kelly J, Yang BB. Design rationale and development approach for pegfilgrastim as a long-acting granulocyte colony-stimulating factor. BioDrugs. 2015; 29:185–98.
Article9. Chen J, Quan H, Gallo P, Menjoge S, Luo X, Tanaka Y, et al. Consistency of treatment effect across regions in multiregional clinical trials, part 1: design considerations. Drug Inf J. 2011; 45:595–602.
Article10. Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009; 361:1045–57.
Article11. Akkerhuis KM, Deckers JW, Boersma E, Harrington RA, Stepinska J, Mahaffey KW, et al. Geographic variability in outcomes within an international trial of glycoprotein IIb/IIIa inhibition in patients with acute coronary syndromes: results from PURSUIT. Eur Heart J. 2000; 21:371–81.
Article12. Kawai N, Chuang-Stein C, Komiyama O, Li Y. An approach to rationalize partitioning sample size into individual regions in a multiregional trial. Drug Inf J. 2008; 42:139–47.13. Ministry of Health, Labour and Welfare. Basic Principles on Global Clinical Trials [Internet]. Tokyo: Pharmaceuticals and Medical Devices Agency;2007. [cited 2023 Jan 16]. Available from: http://www.pmda.go.jp/files/000157900.pdf.14. Barrett JA, Choi J, Lakshmikanthan S, Kim YY, Greene D, Kolli P, et al. Eflapegrastim’s enhancement of efficacy compared with pegfilgrastim in neutropenic rats supports potential for same-day dosing. Exp Hematol. 2020; 92:51–61.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Impact of Primary Prophylactic Pegfilgrastim in Breast Cancer Patients Receiving Adjuvant DocetaxelDoxorubicin-Cyclophosphamide Chemotherapy
- Pegfilgrastim for primary prophylaxis of febrile neutropenia in breast cancer patients undergoing TAC chemotherapy
- Prophylactic Effect of Pegfilgrastim on Febrile Neutropenia in Patients with Non-Hodgikin's Lymphoma
- Predictive Parameters of Febrile Neutropenia and Clinical Significance of G-CSF Receptor Signaling Pathway in the Development of Neutropenia during R-CHOP Chemotherapy with Prophylactic Pegfilgrastim in Patients with Diffuse Large B-Cell Lymphoma
- Clinical utilization of long-acting granulocyte colony-stimulating factor (pegfilgrastim) prophylaxis in breast cancer patients with adjuvant docetaxel-cyclophosphamide chemotherapy