Ann Surg Treat Res.
2018 May;94(5):223-228. 10.4174/astr.2018.94.5.223.
Pegfilgrastim for primary prophylaxis of febrile neutropenia in breast cancer patients undergoing TAC chemotherapy
- Affiliations
-
- 1Department of Surgery, Soonchunhyang University Seoul Hospital, Seoul, Korea.
- 2Department of Surgery, Soonchunhyang University Cheonan Hospital, Cheonan, Korea.
- 3Department of Surgery, Soonchunhyang University Bucheon Hospital, Bucheon, Korea. cwlim@schmc.ac.kr
- KMID: 2410264
- DOI: http://doi.org/10.4174/astr.2018.94.5.223
Abstract
- PURPOSE
Primary prophylaxis with granulocyte colony-stimulating factor can effectively prevent febrile neutropenia (FN) during breast cancer treatment. The aims of this study were to evaluate the incidence of FN and the ANC profile in patients undergoing chemotherapy and pegfilgrastim primary prophylaxis.
METHODS
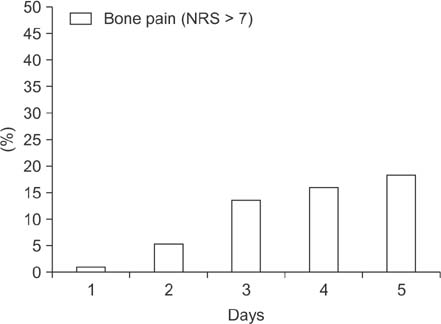
Patients receiving 6 cycles of adjuvant docetaxel, doxorubicin, and cyclophosphamide (TAC) chemotherapy were included in this study. Pegfilgrastim was administered with analgesics 24 hours after treatment. Laboratory tests were performed on day 0 (before chemotherapy) and ANC was measured daily starting day 5 until it were restored to 1,000/mm3. Bone pain was checked via the numeral rating scale (NRS).
RESULTS
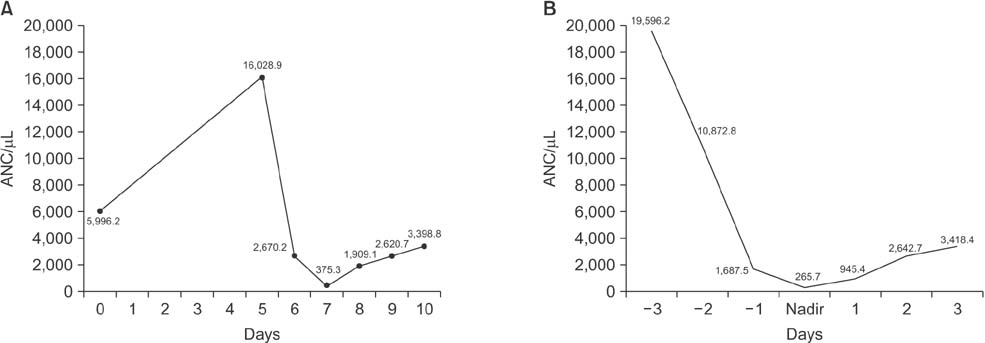
A total of 61 patients and 366 cycles were evaluated. Mean age was 49.2 ± 7.1 years. FN was seen in 5 patients (16.4%) and 12 cycles (3.3%) with pegfilgrastim. Grades 3 and 4 neutropenia was seen in 91.5% of cycles with FN. The ANC nadir was most commonly seen at day 7 and the mean ANC nadir depth was 265.7/m3. Age was negatively correlated with nadir depth (r = −0.137, P = 0.009). Severe pain higher than NRS 7 occurred in less than 20% of patients after the administration of pegfilgrastim.
CONCLUSION
Incidence of FN was low during the chemotherapy by primary prophylaxis with pegfilgrastim. The ANC nadir was seen on day 7 after chemotherapy. Bone pain with pegfilgrastim was well tolerated during TAC chemotherapy.
Keyword
MeSH Terms
Figure
Reference
-
1. Chirivella I, Bermejo B, Insa A, Perez-Fidalgo A, Magro A, Rosello S, et al. Optimal delivery of anthracycline-based chemotherapy in the adjuvant setting improves outcome of breast cancer patients. Breast Cancer Res Treat. 2009; 114:479–484.
Article2. Clark OA, Lyman GH, Castro AA, Clark LG, Djulbegovic B. Colony-stimulating factors for chemotherapy-induced febrile neutropenia: a meta-analysis of randomized controlled trials. J Clin Oncol. 2005; 23:4198–4214.
Article3. Aapro MS, Bohlius J, Cameron DA, Dal Lago L, Donnelly JP, Kearney N, et al. 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer. 2011; 47:8–32.
Article4. Crawford J, Caserta C, Roila F. ESMO Guidelines Working Group. Hematopoietic growth factors: ESMO Clinical Practice Guidelines for the applications. Ann Oncol. 2010; 21:Suppl 5. v248–v251.
Article5. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN Guidelines) [Internet]. Fort Wathington (PA): National Comprehensive Cancer Network;2018. cited 2018 Apr 16. Available from: http://www.nccn.org/professionals/physician_gls/f_ guidelines.asp.6. Aapro M, Crawford J, Kamioner D. Prophylaxis of chemotherapy-induced febrile neutropenia with granulocyte colonystimulating factors: where are we now? Support Care Cancer. 2010; 18:529–541.
Article7. Martin M, Segui MA, Anton A, Ruiz A, Ramos M, Adrover E, et al. Adjuvant docetaxel for high-risk, node-negative breast cancer. N Engl J Med. 2010; 363:2200–2210.8. von Minckwitz G, Kummel S, du Bois A, Eiermann W, Eidtmann H, Gerber B, et al. Pegfilgrastim +/– ciprofloxacin for primary prophylaxis with TAC (docetaxel/doxorubicin/cyclophosphamide) chemotherapy for breast cancer. Results from the GEPARTRIO study. Ann Oncol. 2008; 19:292–298.
Article9. Mitchell S, Li X, Woods M, Garcia J, Hebard-Massey K, Barron R, et al. Comparative effectiveness of granulocyte colony-stimulating factors to prevent febrile neutropenia and related complications in cancer patients in clinical practice: A systematic review. J Oncol Pharm Pract. 2016; 22:702–716.
Article10. Almenar D, Mayans J, Juan O, Bueno JM, Lopez JI, Frau A, et al. Pegfilgrastim and daily granulocyte colony-stimulating factor: patterns of use and neutropenia-related outcomes in cancer patients in Spain--results of the LEARN Study. Eur J Cancer Care (Engl). 2009; 18:280–286.11. Lambertini M, Del Mastro L, Bellodi A, Pronzato P. The five “Ws” for bone pain due to the administration of granulocyte-colony stimulating factors (G-CSFs). Crit Rev Oncol Hematol. 2014; 89:112–128.
Article12. Cortes de Miguel S, Calleja-Hernandez MA, Menjon-Beltran S, Vallejo-Rodriguez I. Granulocyte colony-stimulating factors as prophylaxis against febrile neutropenia. Support Care Cancer. 2015; 23:547–559.
Article13. Lee J, Ahn MH, Jang YH, Lee EJ, Park JH, Rho J, et al. Toxicity and quality of life of Korean breast cancer patients treated with docetaxel-containing chemotherapy without primary G-CSF prophylaxis. Breast Cancer. 2014; 21:670–676.
Article14. Woo HD, Kim HS, Lee JH, Kim HM, Han SW, Kim SY, et al. Toxicity and tolerability study of adjuvant TAC regimen chemotherapy in Korean patients with breast cancer. J Breast Cancer. 2011; 14:Suppl 1. S44–S49.
Article15. Mackey JR, Pienkowski T, Crown J, Sadeghi S, Martin M, Chan A, et al. Long-term outcomes after adjuvant treatment of sequential versus combination docetaxel with doxorubicin and cyclophosphamide in node-positive breast cancer: BCIRG-005 randomized trial. Ann Oncol. 2016; 27:1041–1047.
Article16. Masuda N, Tokuda Y, Nakamura S, Shimazaki R, Ito Y, Tamura K. Dose response of pegfilgrastim in Japanese breast cancer patients receiving six cycles of docetaxel, doxorubicin, and cyclophosphamide therapy: a randomized controlled trial. Support Care Cancer. 2015; 23:2891–2898.
Article17. Kirshner JJ, Heckler CE, Janelsins MC, Dakhil SR, Hopkins JO, Coles C, et al. Prevention of pegfilgrastim-induced bone pain: a phase III double-blind placebo-controlled randomized clinical trial of the university of rochester cancer center clinical community oncology program research base. J Clin Oncol. 2012; 30:1974–1979.
Article18. Leung M, Florendo J, Kano J, Marr-Del Monte T, Higgins B, Myers R, et al. A modified filgrastim regimen does not reduce pain burden compared to pegfilgrastim in women receiving chemotherapy for non-metastatic breast cancer. Support Care Cancer. 2015; 23:1669–1677.
Article19. Klastersky J, Paesmans M, Rubenstein EB, Boyer M, Elting L, Feld R, et al. The Multinational Association for Supportive Care in Cancer risk index: a multinational scoring system for identifying low-risk febrile neutropenic cancer patients. J Clin Oncol. 2000; 18:3038–3051.
Article20. Lyman GH, Kuderer NM, Crawford J, Wolff DA, Culakova E, Poniewierski MS, et al. Predicting individual risk of neutropenic complications in patients receiving cancer chemotherapy. Cancer. 2011; 117:1917–1927.
Article21. Ahn S, Lee YS, Lee JL, Lim KS, Yoon SC. A new prognostic model for chemotherapy-induced febrile neutropenia. Int J Clin Oncol. 2016; 21:46–52.
Article22. Bozcuk H, Yıldız M, Artac M, Kocer M, Kaya C, Ulukal E, et al. A prospectively validated nomogram for predicting the risk of chemotherapy-induced febrile neutropenia: a multicenter study. Support Care Cancer. 2015; 23:1759–1767.
Article23. Lote H, Sharp A, Redana S, Papadimitraki E, Capelan M, Ring A. Febrile neutropenia rates according to body mass index and dose capping in women receiving chemotherapy for early breast cancer. Clin Oncol (R Coll Radiol). 2016; 28:597–603.
Article24. Lee KH, Kim JY, Lee MH, Han HS, Lim JH, Park KU, et al. A randomized, multicenter, phase II/III study to determine the optimal dose and to evaluate the efficacy and safety of pegteograstim (GCPGC) on chemotherapy-induced neutropenia compared to pegfilgrastim in breast cancer patients: KCSG PC10-09. Support Care Cancer. 2016; 24:1709–1717.
Article25. Green MD, Koelbl H, Baselga J, Galid A, Guillem V, Gascon P, et al. A randomized double-blind multicenter phase III study of fixed-dose single-administration pegfilgrastim versus daily filgrastim in patients receiving myelosuppressive chemotherapy. Ann Oncol. 2003; 14:29–35.26. Holmes FA, O'Shaughnessy JA, Vukelja S, Jones SE, Shogan J, Savin M, et al. Blinded, randomized, multicenter study to evaluate single administration pegfilgrastim once per cycle versus daily filgrastim as an adjunct to chemotherapy in patients with high-risk stage II or stage III/IV breast cancer. J Clin Oncol. 2002; 20:727–731.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Impact of Primary Prophylactic Pegfilgrastim in Breast Cancer Patients Receiving Adjuvant DocetaxelDoxorubicin-Cyclophosphamide Chemotherapy
- Clinical utilization of long-acting granulocyte colony-stimulating factor (pegfilgrastim) prophylaxis in breast cancer patients with adjuvant docetaxel-cyclophosphamide chemotherapy
- Prophylactic Effect of Pegfilgrastim on Febrile Neutropenia in Patients with Non-Hodgikin's Lymphoma
- Incidence of Febrile Neutropenia in Advanced Breast Cancer Patients Receiving Adjuvant Docetaxel-Doxorubicin-Cyclophosphamide Chemotherapy in Korea and Its Impact on Prognosis
- Toxicity and Tolerability Study of Adjuvant TAC Regimen Chemotherapy in Korean Patients with Breast Cancer