Ann Surg Treat Res.
2021 Feb;100(2):59-66. 10.4174/astr.2021.100.2.59.
Clinical utilization of long-acting granulocyte colony-stimulating factor (pegfilgrastim) prophylaxis in breast cancer patients with adjuvant docetaxel-cyclophosphamide chemotherapy
- Affiliations
-
- 1Department of Surgery, The Catholic University of Korea, St. Vincent’s Hospital, Suwon, Korea
- 2Department of Nursing, The Catholic University of Korea, St. Vincent’s Hospital, Suwon, Korea
- KMID: 2512477
- DOI: http://doi.org/10.4174/astr.2021.100.2.59
Abstract
- Purpose
Treatment with 4 cycles of docetaxel and cyclophosphamide (TC) in the adjuvant setting is associated with better outcomes than treatment with doxorubicin and cyclophosphamide (AC). However, Western guidelines have indicated that TC confers a high risk (>20%) of febrile neutropenia (FN), while AC confers an intermediate risk (10%–20%) of FN. Threrefore, we evaluated the incidence of FN and the clinical utilization of pegfilgrastim prophylaxis after adjuvant TC chemotherapy.
Methods
We categorized 201 patients who received adjuvant TC chemotherapy into 3 groups according to the method of prophylaxis and compared neutropenic events, other adverse events, and hospital care costs in the 3 groups.
Results
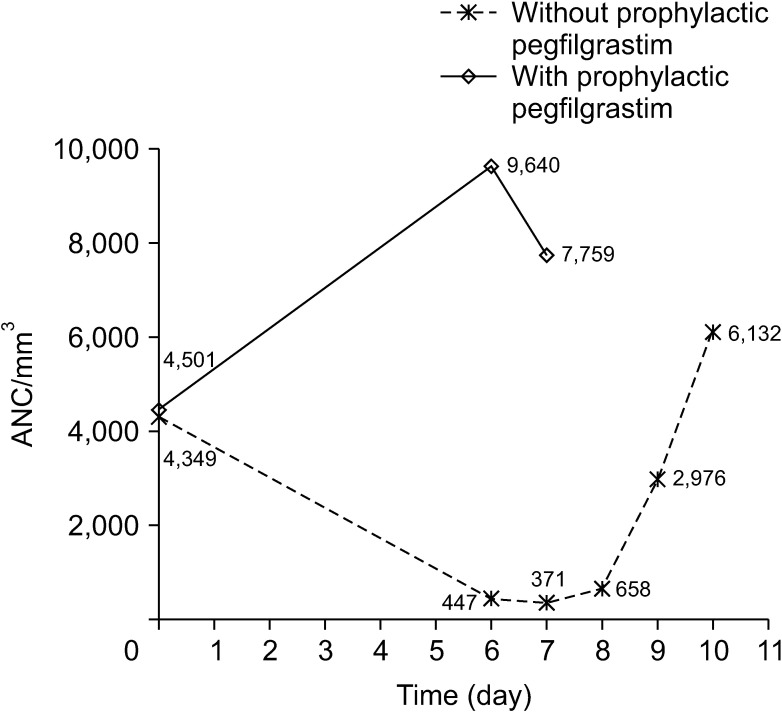
The incidence of grade 4 neutropenia decreased from 93.0% in patients without prophylaxis to 82.4% in those who received secondary prophylaxis and 16.7% in those who received primary prophylaxis. Although the incidence of FN was not different between patients without prophylaxis and patients who received secondary prophylaxis (15.7% and 14.9%), none of the patients who received primary prophylaxis developed FN. Moreover, a decrease in neutropenic events resulted in a significant decrease in the mean duration of neutropenia (2.50 days to 0.08 days, P < 0.001), the risk of hospitalization (29.8% to 2.2%, P < 0.001), and the mean total hospital care cost for all chemotherapy cycles (790.80 to 486.00 US dollars, P < 0.001).
Conclusion
The use of pegfilgrastim prophylaxis during adjuvant TC chemotherapy is associated with significant decreases in the incidence of neutropenic events, hospitalization, and hospital care cost compared to those seen in patients without prophylaxis.
Figure
Reference
-
1. Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005; 365:1687–1717. PMID: 15894097.2. Smith LA, Cornelius VR, Plummer CJ, Levitt G, Verrill M, Canney P, et al. Cardiotoxicity of anthracycline agents for the treatment of cancer: systematic review and meta-analysis of randomised controlled trials. BMC Cancer. 2010; 10:337. PMID: 20587042.
Article3. Pal SK, Childs BH, Pegram M. Emergence of nonanthracycline regimens in the adjuvant treatment of breast cancer. Breast Cancer Res Treat. 2010; 119:25–32. PMID: 19795206.
Article4. Smith RE, Bryant J, DeCillis A, Anderson S. National Surgical Adjuvant Breast and Bowel Project Experience. Acute myeloid leukemia and myelodysplastic syndrome after doxorubicin-cyclophosphamide adjuvant therapy for operable breast cancer: the National Surgical Adjuvant Breast and Bowel Project experience. J Clin Oncol. 2003; 21:1195–1204. PMID: 12663705.
Article5. Jones SE, Savin MA, Holmes FA, O'Shaughnessy JA, Blum JL, Vukelja S, et al. Phase III trial comparing doxorubicin plus cyclophosphamide with docetaxel plus cyclophosphamide as adjuvant therapy for operable breast cancer. J Clin Oncol. 2006; 24:5381–5387. PMID: 17135639.
Article6. Giordano SH, Lin YL, Kuo YF, Hortobagyi GN, Goodwin JS. Decline in the use of anthracyclines for breast cancer. J Clin Oncol. 2012; 30:2232–2239. PMID: 22614988.
Article7. Jones S, Holmes FA, O'Shaughnessy J, Blum JL, Vukelja SJ, McIntyre KJ, et al. Docetaxel with cyclophosphamide is associated with an overall survival benefit compared with doxorubicin and cyclophosphamide: 7-year follow-up of us oncology research trial 9735. J Clin Oncol. 2009; 27:1177–1183. PMID: 19204201.
Article8. Blum JL, Flynn PJ, Yothers G, Asmar L, Geyer CE Jr, Jacobs SA, et al. Anthracyclines in early breast cancer: the ABC Trials-USOR 06-090, NSABP B-46-I/USOR 07132, and NSABP B-49 (NRG Oncology). J Clin Oncol. 2017; 35:2647–2655. PMID: 28398846.
Article9. Nitz U, Gluz O, Clemens M, Malter W, Reimer T, Nuding B, et al. West German Study PlanB trial: adjuvant four cycles of epirubicin and cyclophosphamide plus docetaxel versus six cycles of docetaxel and cyclophosphamide in HER2-negative early breast cancer. J Clin Oncol. 2019; 37:799–808. PMID: 30785826.
Article10. Dale DC. Colony-stimulating factors for the management of neutropenia in cancer patients. Drugs. 2002; 62 Suppl 1:1–15.
Article11. Kuderer NM, Dale DC, Crawford J, Lyman GH. Impact of primary prophylaxis with granulocyte colony-stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: a systemat ic review. J Cl in Oncol. 2007; 25:3158–3167.12. National Comprehensive Cancer Networt (NCCN). Clinical practice guidelines in oncology for hematopoietic growth factors (NCCN Guidelines version 2.2020) [Internet]. Fort Washington, PA: NCCN;2020. cited 2020 Apr 7. Available from: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.13. Younis T, Rayson D, Thompson K. Primary G-CSF prophylaxis for adjuvant TC or FEC-D chemotherapy outside of clinical trial settings: a systematic review and meta-analysis. Support Care Cancer. 2012; 20:2523–2530. PMID: 22252548.
Article14. Ishiguro H, Masuda N, Sato N, Higaki K, Morimoto T, Yanagita Y, et al. A randomized study comparing docetaxel/cyclophosphamide (TC), 5-fluorouracil/epirubicin/cyclophosphamide (FEC) followed by TC, and TC followed by FEC for patients with hormone receptor-positive HER2-negative primary breast cancer. Breast Cancer Res Treat. 2020; 180:715–724. PMID: 32170634.
Article15. Weycker D, Barron R, Edelsberg J, Kartashov A, Lyman GH. Incidence of reduced chemotherapy relative dose intensity among women with early stage breast cancer in US clinical practice. Breast Cancer Res Treat. 2012; 133:301–310. PMID: 22270932.
Article16. Fernandes R, Mazzarello S, Stober C, Vandermeer L, Dudani S, Ibrahim MF, et al. Optimal primary febrile neutropenia prophylaxis for patients receiving docetaxel-cyclophosphamide chemotherapy for breast cancer: a systematic review. Breast Cancer Res Treat. 2017; 161:1–10. PMID: 27783280.
Article17. Li Y, Family L, Yang SJ, Klippel Z, Page JH, Chao C. Risk of febrile neutropenia associated with select myelosuppressive chemotherapy regimens in a large community-based oncology practice. J Natl Compr Canc Netw. 2017; 15:1122–1130. PMID: 28874597.
Article18. Baker SD, Li J, ten Tije AJ, Figg WD, Graveland W, Verweij J, et al. Relationship of systemic exposure to unbound docetaxel and neut ropenia. Cl in Pharmacol Ther. 2005; 77:43–53.19. Hor SY, Lee SC, Wong CI, Lim YW, Lim RC, Wang LZ, et al. PXR, CAR and HNF4alpha genotypes and their association with pharmacokinetics and pharmacodynamics of docetaxel and doxorubicin in Asian patients. Pharmacogenomics J. 2008; 8:139–146. PMID: 17876342.20. Onoue H, Yano I, Tanaka A, Itohara K, Hanai A, Ishiguro H, et al. Significant effect of age on docetaxel pharmacokinetics in Japanese female breast cancer patients by using the population modeling approach. Eur J Clin Pharmacol. 2016; 72:703–710. PMID: 26905999.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pegfilgrastim for primary prophylaxis of febrile neutropenia in breast cancer patients undergoing TAC chemotherapy
- Clinical Impact of Primary Prophylactic Pegfilgrastim in Breast Cancer Patients Receiving Adjuvant DocetaxelDoxorubicin-Cyclophosphamide Chemotherapy
- Incidence of Febrile Neutropenia in Advanced Breast Cancer Patients Receiving Adjuvant Docetaxel-Doxorubicin-Cyclophosphamide Chemotherapy in Korea and Its Impact on Prognosis
- Prophylactic Effect of Pegfilgrastim on Febrile Neutropenia in Patients with Non-Hodgikin's Lymphoma
- Eflapegrastim versus Pegfilgrastim for Chemotherapy-Induced Neutropenia in Korean and Asian Patients with Early Breast Cancer: Results from the Two Phase III ADVANCE and RECOVER Studies