Cancer Res Treat.
2022 Oct;54(4):1256-1267. 10.4143/crt.2021.944.
Predictive Parameters of Febrile Neutropenia and Clinical Significance of G-CSF Receptor Signaling Pathway in the Development of Neutropenia during R-CHOP Chemotherapy with Prophylactic Pegfilgrastim in Patients with Diffuse Large B-Cell Lymphoma
- Affiliations
-
- 1Division of Hematology-Oncology, Department of Internal Medicine, Biochemical Research Institution, Pusan National University Hospital, Pusan National University School of Medicine, Busan, Korea
- 2Department of Biological Sciences, Pusan National University, Busan, Korea
- 3Department of Internal Medicine, Dong-A University College of Medicine, Busan, Korea
- 4Division of Hematology-Oncology, Department of Internal Medicine, Ulsan University Hospital, Ulsan, Korea
- KMID: 2534204
- DOI: http://doi.org/10.4143/crt.2021.944
Abstract
- Purpose
Pegfilgrastim is widely used to prevent chemotherapy-induced neutropenia (CIN) and febrile neutropenia (FN) in patients with diffuse large B-cell lymphoma (DLBCL). We investigated the predictive factors affecting CIN and FN incidence in patients with DLBCL receiving rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) chemotherapy with pegfilgrastim and conducted experiments to find reason for the occurrence of CIN even when pegfilgrastim was used.
Materials and Methods
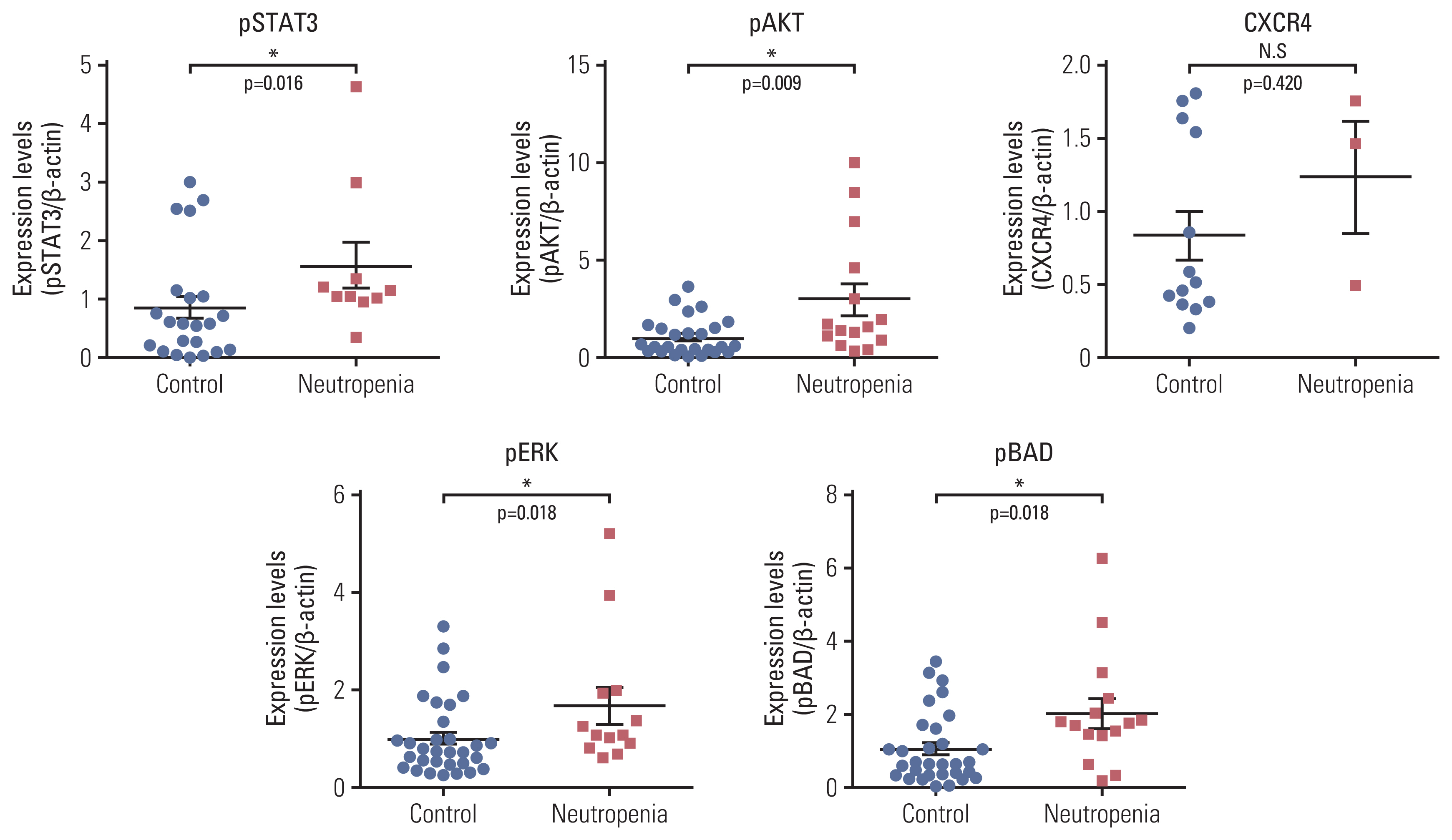
We reviewed the CIN and FN events of 200 patients with DLBCL. Based on these data, we investigate the association with predictive factor and the levels of granulocyte-colony stimulating factor (G-CSF) receptor signaling pathway markers (pSTAT3, pAKT, pERK1/2, pBAD, and CXCR4) in bone marrow (BM) samples isolated from patients with DLBCL.
Results
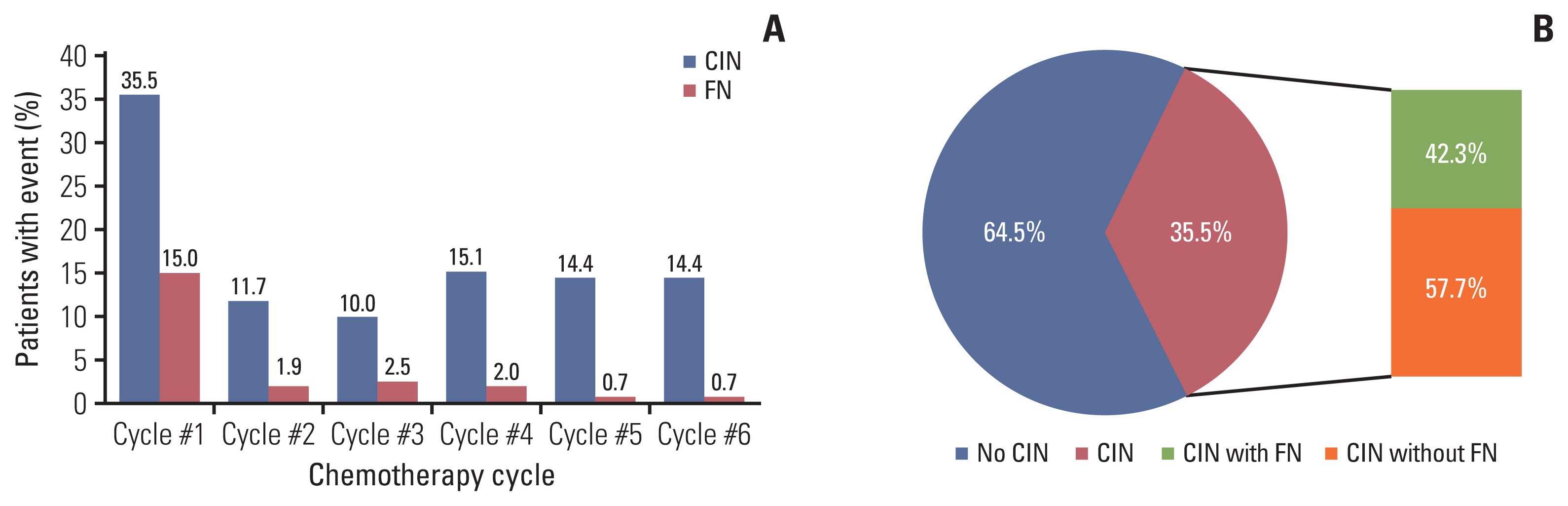
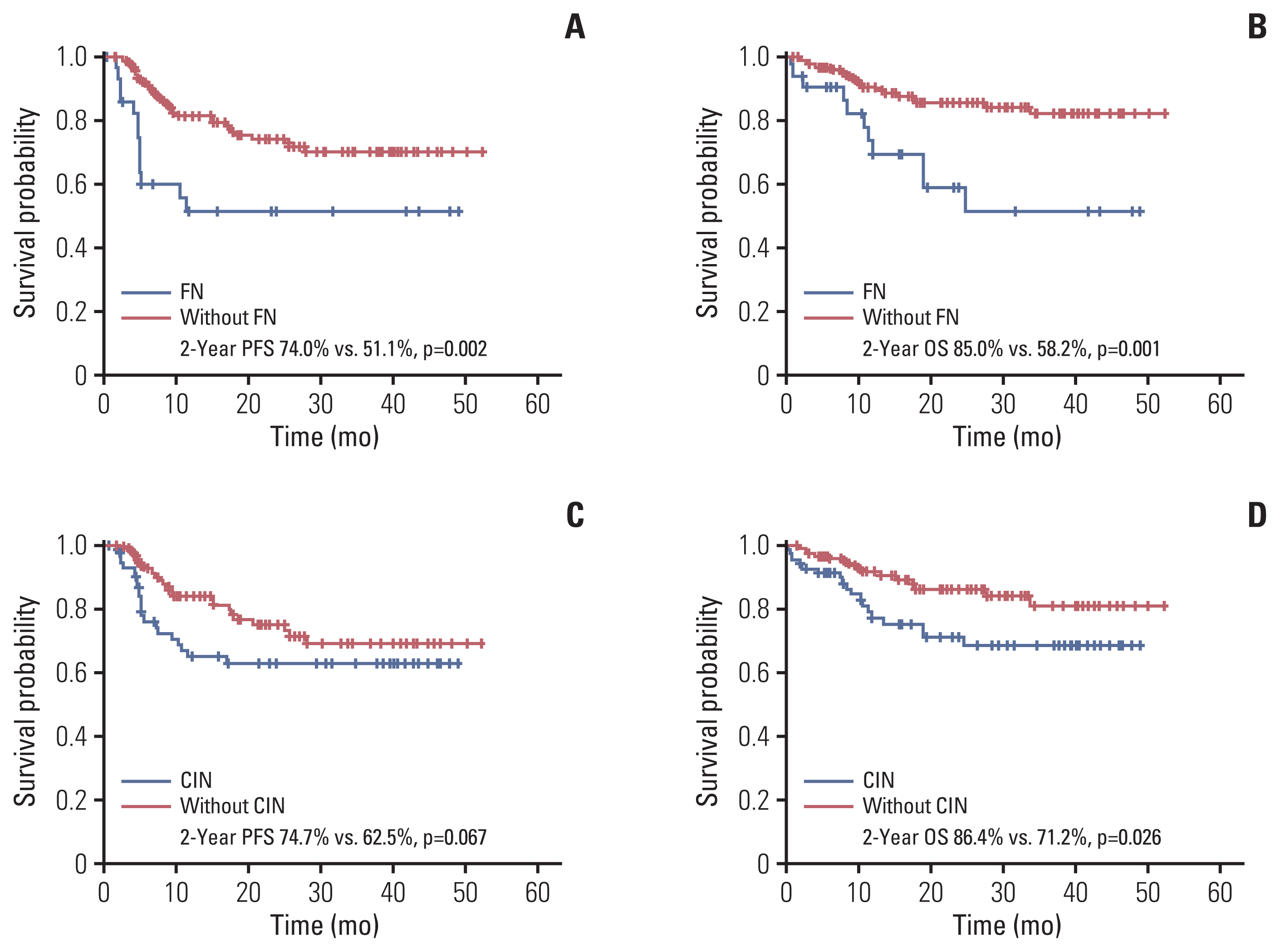
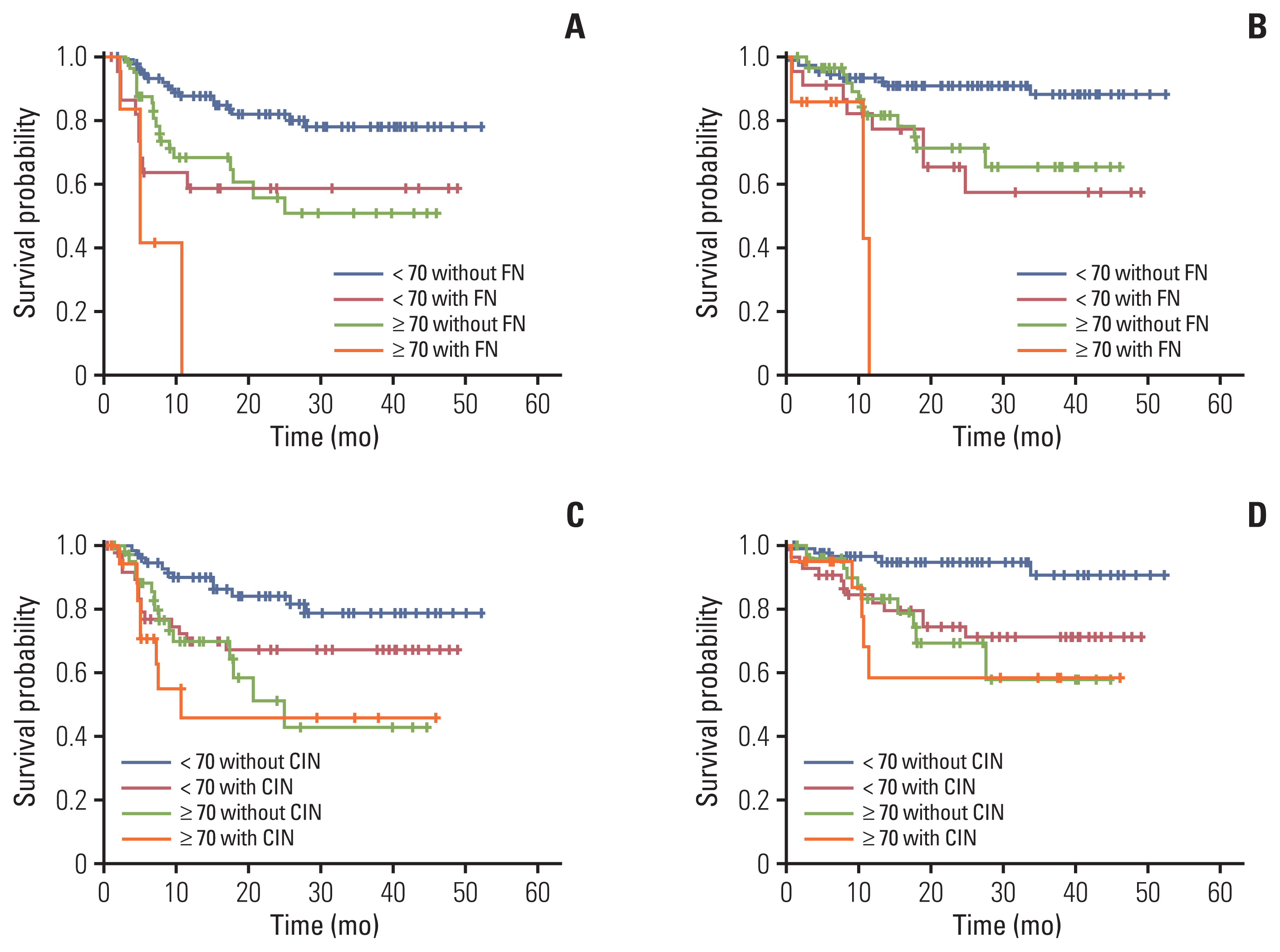
FN was significantly associated with stage III/IV (hazard ratio [HR], 12.74) and low serum albumin levels (HR, 3.87). Additionally, patients with FN had lower progression-free survival (PFS; 2-year PFS, 51.1 % vs. 74.0%) and overall survival (OS; 2-year OS, 58.2% vs. 85.0%) compared to those without FN. The occurrence of CIN was associated with overexpression of G-CSF receptor signaling pathway markers, and expression levels of these markers were upregulated in BM cells co-cultured with DLBCL cells. The rate of neutrophil apoptosis was also higher in neutrophils co-cultured with DLBCL cells and was further promoted by treatment with doxorubicin.
Conclusion
Our findings suggest that high DLBCL burden may alter the BM environment and G-CSF receptor signaling pathway, even in chemotherapy-naïve state, which may increase CIN frequency during R-CHOP chemotherapy.
Figure
Reference
-
References
1. Morton LM, Turner JJ, Cerhan JR, Linet MS, Treseler PA, Clarke CA, et al. Proposed classification of lymphoid neoplasms for epidemiologic research from the Pathology Working Group of the International Lymphoma Epidemiology Consortium (InterLymph). Blood. 2007; 110:695–708.2. Lee L, Crump M, Khor S, Hoch JS, Luo J, Bremner K, et al. Impact of rituximab on treatment outcomes of patients with diffuse large b-cell lymphoma: a population-based analysis. Br J Haematol. 2012; 158:481–8.3. Lindenmeyer LP, Hegele V, Caregnato JP, Wust D, Grazziotin L, Stoll P. Follow-up of patients receiving rituximab for diffuse large B cell lymphoma: an overview of systematic reviews. Ann Hematol. 2013; 92:1451–9.4. Crawford J, Dale DC, Lyman GH. Chemotherapy-induced neutropenia: risks, consequences, and new directions for its management. Cancer. 2004; 100:228–37.5. Kuderer NM, Dale DC, Crawford J, Cosler LE, Lyman GH. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer. 2006; 106:2258–66.6. Bosly A, Bron D, Van Hoof A, De Bock R, Berneman Z, Ferrant A, et al. Achievement of optimal average relative dose intensity and correlation with survival in diffuse large B-cell lymphoma patients treated with CHOP. Ann Hematol. 2008; 87:277–83.7. Lyman GH. Impact of chemotherapy dose intensity on cancer patient outcomes. J Natl Compr Canc Netw. 2009; 7:99–108.8. Aapro MS, Bohlius J, Cameron DA, Dal Lago L, Donnelly JP, Kearney N, et al. 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer. 2011; 47:8–32.9. National Comprehensive Cancer Network. Hematopoietic growth factors (version 4, 2021) [Internet]. Plymouth Meeting, PA: National Comprehensive Cancer Network;c2021. [cited 2021 May 20]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/growthfactors.pdf .10. Maher DW, Lieschke GJ, Green M, Bishop J, Stuart-Harris R, Wolf M, et al. Filgrastim in patients with chemotherapy-induced febrile neutropenia: a double-blind, placebo-controlled trial. Ann Intern Med. 1994; 121:492–501.11. Cooper KL, Madan J, Whyte S, Stevenson MD, Akehurst RL. Granulocyte colony-stimulating factors for febrile neutropenia prophylaxis following chemotherapy: systematic review and meta-analysis. BMC Cancer. 2011; 11:404.12. Tidow N, Welte K. Advances in understanding postreceptor signaling in response to granulocyte colony-stimulating factor. Curr Opin Hematol. 1997; 4:171–5.13. Demetri GD, Griffin JD. Granulocyte colony-stimulating factor and its receptor. Blood. 1991; 78:2791–808.14. Yang BB, Kido A. Pharmacokinetics and pharmacodynamics of pegfilgrastim. Clin Pharmacokinet. 2011; 50:295–306.15. Green MD, Koelbl H, Baselga J, Galid A, Guillem V, Gascon P, et al. A randomized double-blind multicenter phase III study of fixed-dose single-administration pegfilgrastim versus daily filgrastim in patients receiving myelosuppressive chemotherapy. Ann Oncol. 2003; 14:29–35.16. Holmes FA, O’Shaughnessy JA, Vukelja S, Jones SE, Shogan J, Savin M, et al. Blinded, randomized, multicenter study to evaluate single administration pegfilgrastim once per cycle versus daily filgrastim as an adjunct to chemotherapy in pati-ents with high-risk stage II or stage III/IV breast cancer. J Clin Oncol. 2002; 20:727–31.17. Brusamolino E, Rusconi C, Montalbetti L, Gargantini L, Uziel L, Pinotti G, et al. Dose-dense R-CHOP-14 supported by pegfilgrastim in patients with diffuse large B-cell lymphoma: a phase II study of feasibility and toxicity. Haematologica. 2006; 91:496–502.18. Yokoyama M, Kusano Y, Takahashi A, Inoue N, Ueda K, Nishimura N, et al. Incidence and risk factors of febrile neutropenia in patients with non-Hodgkin B-cell lymphoma receiving R-CHOP in a single center in Japan. Support Care Cancer. 2017; 25:3313–20.19. Lyman GH, Delgado DJ. Risk and timing of hospitalization for febrile neutropenia in patients receiving CHOP, CHOP-R, or CNOP chemotherapy for intermediate-grade non-Hodgkin lymphoma. Cancer. 2003; 98:2402–9.20. Lyman GH, Morrison VA, Dale DC, Crawford J, Delgado DJ, Fridman M, et al. Risk of febrile neutropenia among patients with intermediate-grade non-Hodgkin’s lymphoma receiving CHOP chemotherapy. Leuk Lymphoma. 2003; 44:2069–76.21. Morrison VA, Weller EA, Habermann TM, Li S, Fisher RI, Cheson BD, et al. Patterns of growth factor usage and febrile neutropenia among older patients with diffuse large B-cell non-Hodgkin lymphoma treated with CHOP or R-CHOP: the Intergroup experience (CALGB 9793; ECOG-SWOG 4494). Leuk Lymphoma. 2017; 58:1814–22.22. Ray-Coquard I, Borg C, Bachelot T, Sebban C, Philip I, Clapisson G, et al. Baseline and early lymphopenia predict for the risk of febrile neutropenia after chemotherapy. Br J Cancer. 2003; 88:181–6.23. Lyman GH, Abella E, Pettengell R. Risk factors for febrile neutropenia among patients with cancer receiving chemotherapy: a systematic review. Crit Rev Oncol Hematol. 2014; 90:190–9.24. Lee DG, Kim SH, Kim SY, Kim CJ, Park WB, Song YG, et al. Evidence-based guidelines for empirical therapy of neutropenic fever in Korea. Korean J Intern Med. 2011; 26:220–52.25. Choi YW, Jeong SH, Ahn MS, Lee HW, Kang SY, Choi JH, et al. Patterns of neutropenia and risk factors for febrile neutropenia of diffuse large B-cell lymphoma patients treated with rituximab-CHOP. J Korean Med Sci. 2014; 29:1493–500.26. Fukunaga R, Ishizaka-Ikeda E, Nagata S. Growth and differentiation signals mediated by different regions in the cytoplasmic domain of granulocyte colony-stimulating factor receptor. Cell. 1993; 74:1079–87.27. Santini V, Scappini B, Indik ZK, Gozzini A, Ferrini PR, Schreiber AD. The carboxy-terminal region of the granulocyte colony-stimulating factor receptor transduces a phagocytic signal. Blood. 2003; 101:4615–22.28. Palande K, Meenhuis A, Jevdjovic T, Touw IP. Scratching the surface: signaling and routing dynamics of the CSF3 receptor. Front Biosci (Landmark Ed). 2013; 18:91–105.29. Ok CY, Chen J, Xu-Monette ZY, Tzankov A, Manyam GC, Li L, et al. Clinical implications of phosphorylated STAT3 expression in de novo diffuse large B-cell lymphoma. Clin Cancer Res. 2014; 20:5113–23.30. Hong JY, Hong ME, Choi MK, Kim YS, Chang W, Maeng CH, et al. The impact of activated p-AKT expression on clinical outcomes in diffuse large B-cell lymphoma: a clinicopathological study of 262 cases. Ann Oncol. 2014; 25:182–8.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prophylactic Effect of Pegfilgrastim on Febrile Neutropenia in Patients with Non-Hodgikin's Lymphoma
- Patterns of Neutropenia and Risk Factors for Febrile Neutropenia of Diffuse Large B-Cell Lymphoma Patients Treated with Rituximab-CHOP
- Utility Analysis for Pegfilgrastim in DLBCL Patients on R-CHOP Regimen
- Clinical Impact of Primary Prophylactic Pegfilgrastim in Breast Cancer Patients Receiving Adjuvant DocetaxelDoxorubicin-Cyclophosphamide Chemotherapy
- Risk factors for neutropenic fever in non-Hodgkin’s lymphoma patients with primary granulocyte colony-stimulating factor prophylaxis