J Korean Med Sci.
2023 Apr;38(14):e101. 10.3346/jkms.2023.38.e101.
Evaluation of Vancomycin TDM Strategies: Prediction and Prevention of Kidney Injuries Based on Vancomycin TDM Results
- Affiliations
-
- 1Department of Clinical Pharmacology and Therapeutics, Seoul National University Hospital, Seoul National University College of Medicine Seoul, Korea
- 2Department of Pharmacy, Seoul National University Bundang Hospital, Seongnam, Korea
- 3Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- 4Department of Laboratory Medicine, Seoul National University Bundang Hospital, Seongnam, Korea
- 5Department of Clinical Pharmacology and Therapeutics, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- KMID: 2541546
- DOI: http://doi.org/10.3346/jkms.2023.38.e101
Abstract
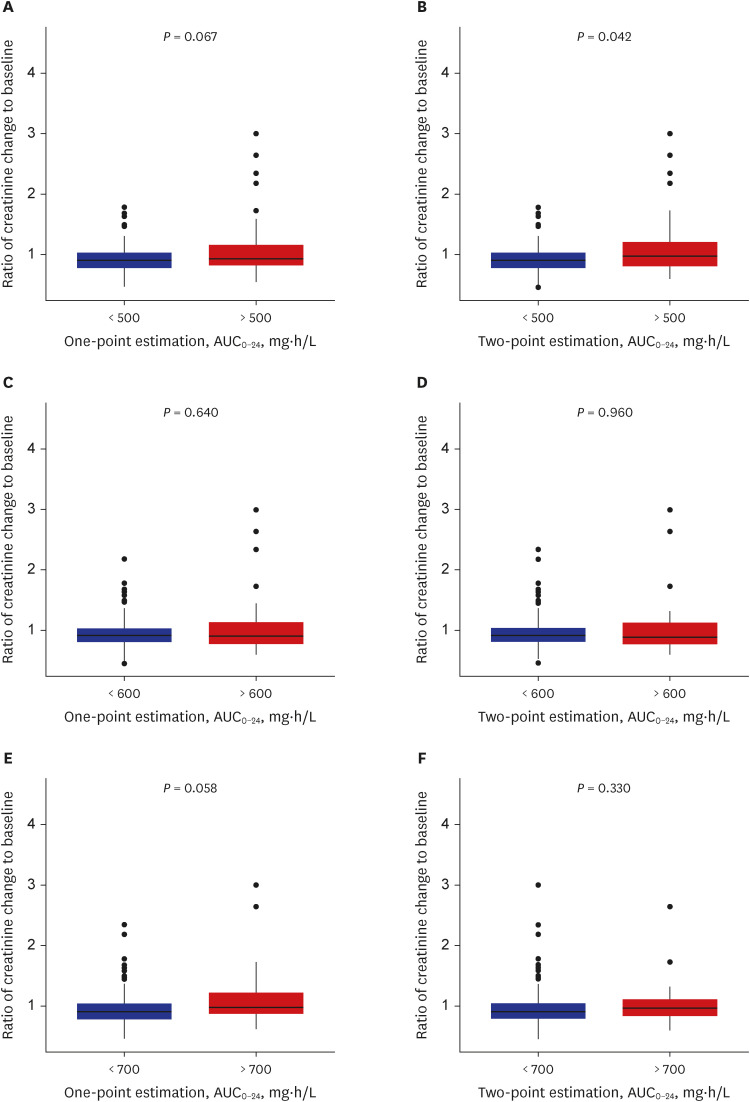
- The current guidelines for therapeutic drug monitoring (TDM) of vancomycin suggest a target 24-hour area under the curve (AUC 0-24 ) of 400 to 600 mg*h/L for serious methicillinresistant Staphylococcus aureus infections. In this study, the predictabilities of acute kidney injury (AKI) of various TDM target parameters, target levels, and sampling methods were evaluated in patients who underwent TDM from January 2020 to December 2020. The AUC 0-24 and trough values were calculated by both one- and two-point sampling methods, and were evaluated for the predictability of AKI. Among the AUC 0-24 cutoff comparisons, the threshold value of 500 mg*h/L in the two sampling methods was statistically significant (P = 0.042) when evaluated for the predictability of AKI. Analysis by an receiver operating characteristic curve estimated an AUC 0-24 cutoff value of 563.45 mg*h/L as a predictor of AKI, and was proposed as the upper limit of TDM target.
Figure
Cited by 1 articles
-
Survey on the Current Status of Therapeutic Drug Monitoring and Vancomycin Pharmacokinetic Consultation Service in Clinical Laboratories in Korea
Mikyoung Park, Hyun-Ki Kim, Jong Do Seo, Tae-Dong Jeong, Misuk Ji
Lab Med Online. 2025;15(1):58-69. doi: 10.47429/lmo.2025.15.1.58.
Reference
-
1. Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011; 52(3):285–292. PMID: 21217178.
Article2. Choi SH, Lee J, Jung J, Kim ES, Kim MJ, Chong YP, et al. A longitudinal study of adult patients with Staphylococcus aureus bacteremia over 11 years in Korea. J Korean Med Sci. 2021; 36(16):e104. PMID: 33904260.
Article3. Kim YS, Kiem S, Yun HJ, Jung SI, Oh WS, Kim SW, et al. Efficacy of vancomycin-beta-lactam combinations against heterogeneously vancomycin-resistant Staphylococcus aureus (hetero-VRSA). J Korean Med Sci. 2003; 18(3):319–324. PMID: 12808315.
Article4. Sinha Ray A, Haikal A, Hammoud KA, Yu AS. Vancomycin and the risk of AKI: a systematic review and meta-analysis. Clin J Am Soc Nephrol. 2016; 11(12):2132–2140. PMID: 27895134.5. Yoo RN, Kim SH, Lee J. Impact of initial vancomycin trough concentration on clinical and microbiological outcomes of methicillin-resistant Staphylococcus aureus bacteremia in children. J Korean Med Sci. 2017; 32(1):22–28. PMID: 27914127.6. Stoessel AM, Hale CM, Seabury RW, Miller CD, Steele JM. The impact of AUC-based monitoring on pharmacist-directed vancomycin dose adjustments in complicated methicillin-resistant Staphylococcus aureus infection. J Pharm Pract. 2019; 32(4):442–446. PMID: 29554847.7. Wong-Beringer A, Joo J, Tse E, Beringer P. Vancomycin-associated nephrotoxicity: a critical appraisal of risk with high-dose therapy. Int J Antimicrob Agents. 2011; 37(2):95–101. PMID: 21130609.
Article8. Rybak MJ, Le J, Lodise TP, Levine DP, Bradley JS, Liu C, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2020; 77(11):835–864. PMID: 32191793.
Article9. Gameiro J, Agapito Fonseca J, Jorge S, Lopes JA. Acute kidney injury definition and diagnosis: a narrative review. J Clin Med. 2018; 7(10):307. PMID: 30274164.
Article10. Na KR, Kim HR, Ham Y, Choi DE, Lee KW, Moon JY, et al. Acute kidney injury and kidney damage in COVID-19 patients. J Korean Med Sci. 2020; 35(28):e257. PMID: 32686373.
Article11. Filippone EJ, Kraft WK, Farber JL. The nephrotoxicity of vancomycin. Clin Pharmacol Ther. 2017; 102(3):459–469. PMID: 28474732.
Article12. Kato H, Hagihara M, Okudaira M, Asai N, Koizumi Y, Yamagishi Y, et al. Systematic review and meta-analysis to explore optimal therapeutic range of vancomycin trough level for infected paediatric patients with gram-positive pathogens to reduce mortality and nephrotoxicity risk. Int J Antimicrob Agents. 2021; 58(2):106393. PMID: 34174409.
Article13. Suzuki A, Hamada Y, Ikeda H, Tanaka H, Yanagihara M, Namiki M, et al. Comparison of trough concentration and area under the curve of vancomycin associated with the incidence of nephrotoxicity and predictors of a high trough level. J Infect Chemother. 2021; 27(3):455–460. PMID: 33144145.
Article14. van Hal SJ, Paterson DL, Lodise TP. Systematic review and meta-analysis of vancomycin-induced nephrotoxicity associated with dosing schedules that maintain troughs between 15 and 20 milligrams per liter. Antimicrob Agents Chemother. 2013; 57(2):734–744. PMID: 23165462.
Article15. Chavada R, Ghosh N, Sandaradura I, Maley M, Van Hal SJ. Establishment of an AUC0-24 threshold for nephrotoxicity is a step towards individualized vancomycin dosing for methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2017; 61(5):e02535-16. PMID: 28242672.
Article16. Bellos I, Daskalakis G, Pergialiotis V. Relationship of vancomycin trough levels with acute kidney injury risk: an exposure-toxicity meta-analysis. J Antimicrob Chemother. 2020; 75(10):2725–2734. PMID: 32417905.
Article17. Neely MN, Youn G, Jones B, Jelliffe RW, Drusano GL, Rodvold KA, et al. Are vancomycin trough concentrations adequate for optimal dosing? Antimicrob Agents Chemother. 2014; 58(1):309–316. PMID: 24165176.18. Ingram PR, Lye DC, Tambyah PA, Goh WP, Tam VH, Fisher DA. Risk factors for nephrotoxicity associated with continuous vancomycin infusion in outpatient parenteral antibiotic therapy. J Antimicrob Chemother. 2008; 62(1):168–171. PMID: 18334494.
Article19. Chattaweelarp T, Changpradub D, Punyawudho B, Thunyaharn S, Santimaleeworagun W. Is early monitoring better? Impact of early vancomycin exposure on treatment outcomes and nephrotoxicity in patients with methicillin-resistant Staphylococcus aureus infections. Antibiotics (Basel). 2020; 9(10):672. PMID: 33020463.
Article20. Lodise TP, Patel N, Lomaestro BM, Rodvold KA, Drusano GL. Relationship between initial vancomycin concentration-time profile and nephrotoxicity among hospitalized patients. Clin Infect Dis. 2009; 49(4):507–514. PMID: 19586413.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Trough-Based and Area Under the Curve-Based Therapeutic Drug Monitoring of Vancomycin: An In Silico Study
- Comparison of Trough-Based and Area Under the Curve-Based Therapeutic Drug Monitoring of Vancomycin: An In Silico Study
- Therapeutic Drug Monitoring (TDM) of Antimicrobial Agents
- Therapeutic drug monitoring of vancomycin in a patient with Duchenne muscular dystrophy (DMD): A case report
- Vancomycin and Aminoglycoside Antibiotic Drug Concentration Measurement: Current Status in Clinical Laboratories in Korea