Lab Med Online.
2020 Oct;10(4):265-275. 10.47429/lmo.2020.10.4.265.
Vancomycin and Aminoglycoside Antibiotic Drug Concentration Measurement: Current Status in Clinical Laboratories in Korea
- Affiliations
-
- 1Department of Laboratory Medicine and Genetics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2512265
- DOI: http://doi.org/10.47429/lmo.2020.10.4.265
Abstract
- Background
Therapeutic drug monitoring (TDM) is clinically recommended for vancomycin and aminoglycoside antibiotics owing to their narrow therapeutic range and nephrotoxicity at high concentrations in the blood. This study was conducted to investigate the current status of TDM of vancomycin and aminoglycosides in Korean clinical laboratories.
Methods
Ten organizations participated in this survey. Vancomycin, amikacin, gentamicin, and tobramycin were prepared in three samples of five or six different concentrations. Data from each institution were calculated for the mean, standard deviation, within-day, between-day, and within-laboratory precision. The results from each institution were compared in various ways.
Results
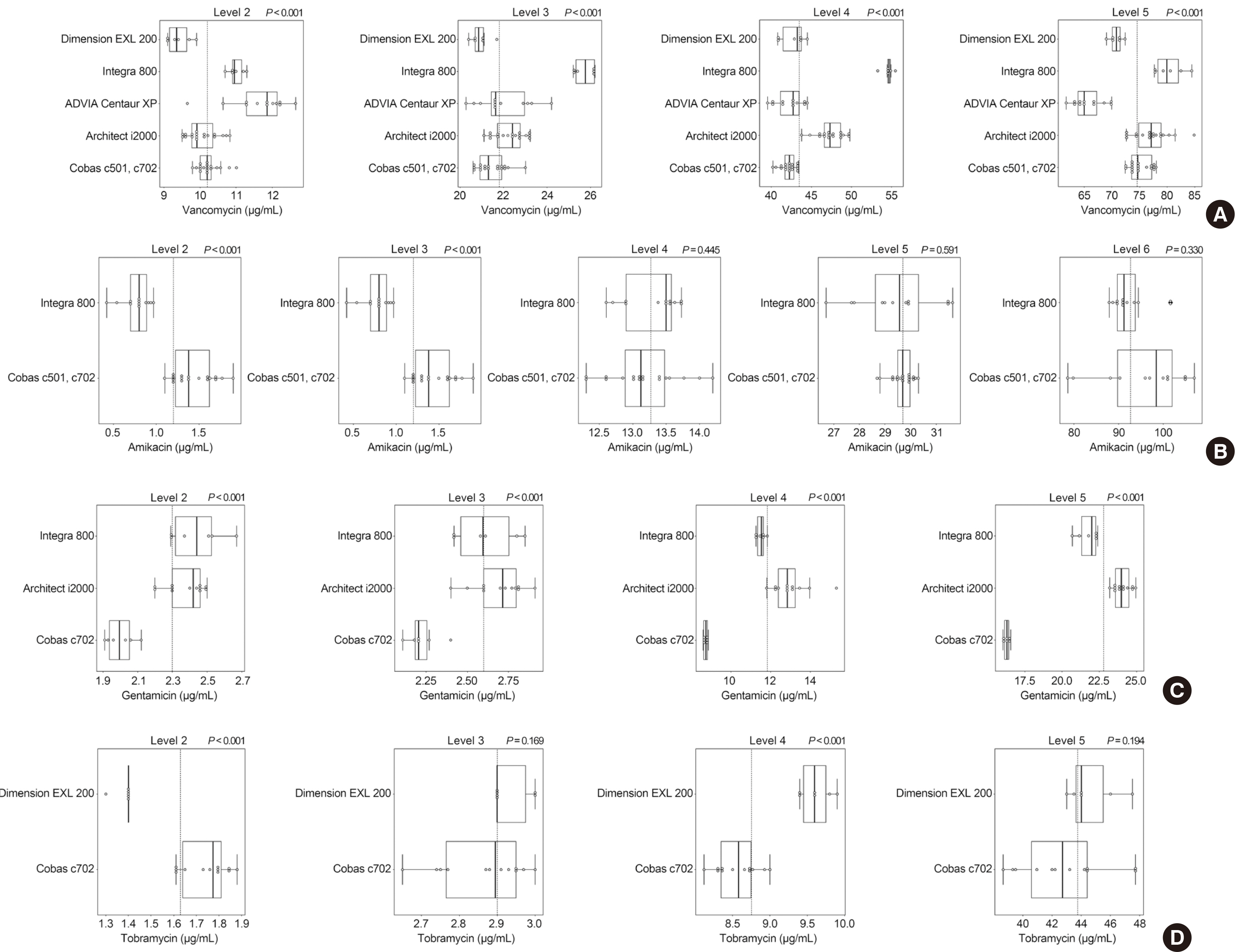
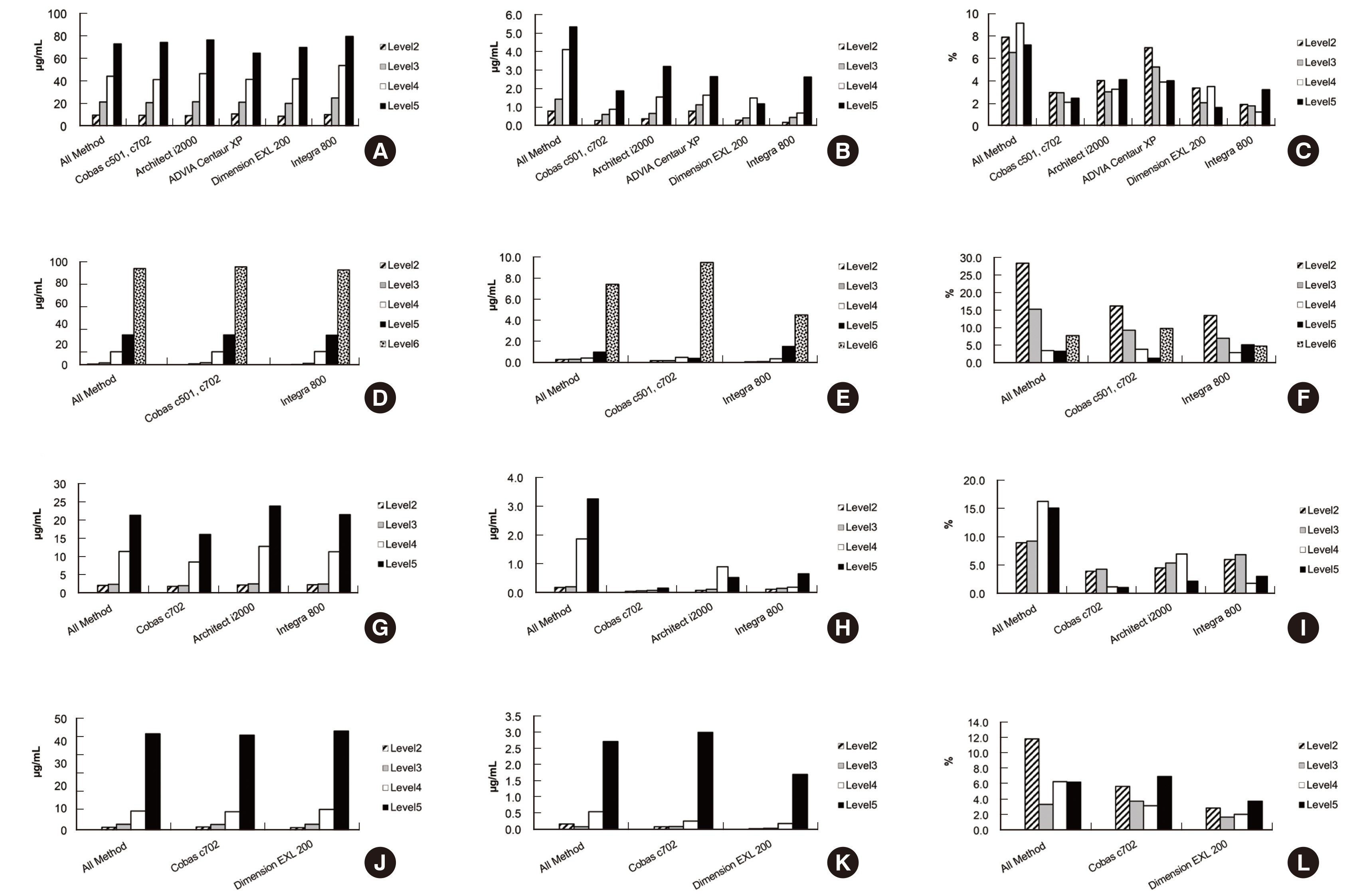
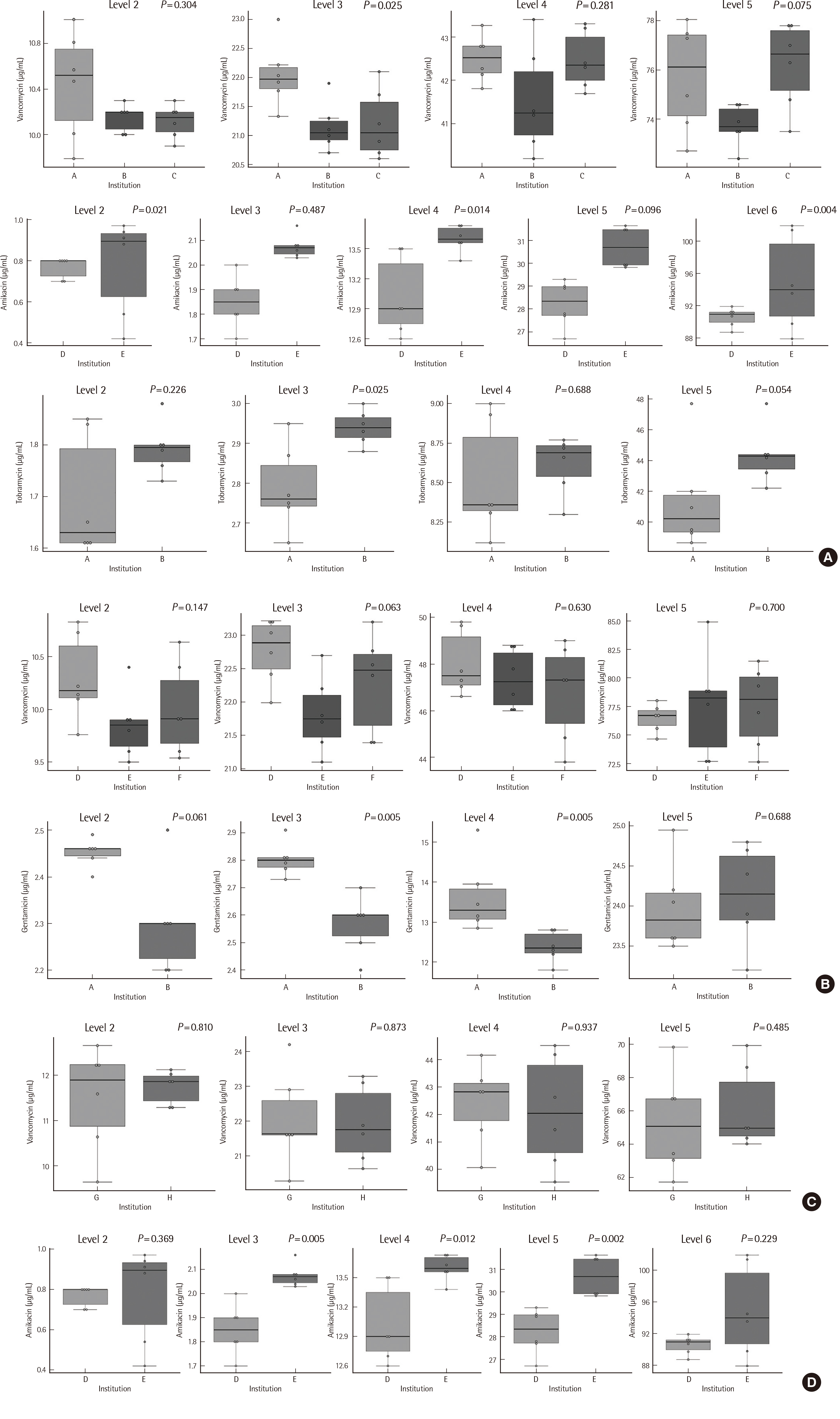
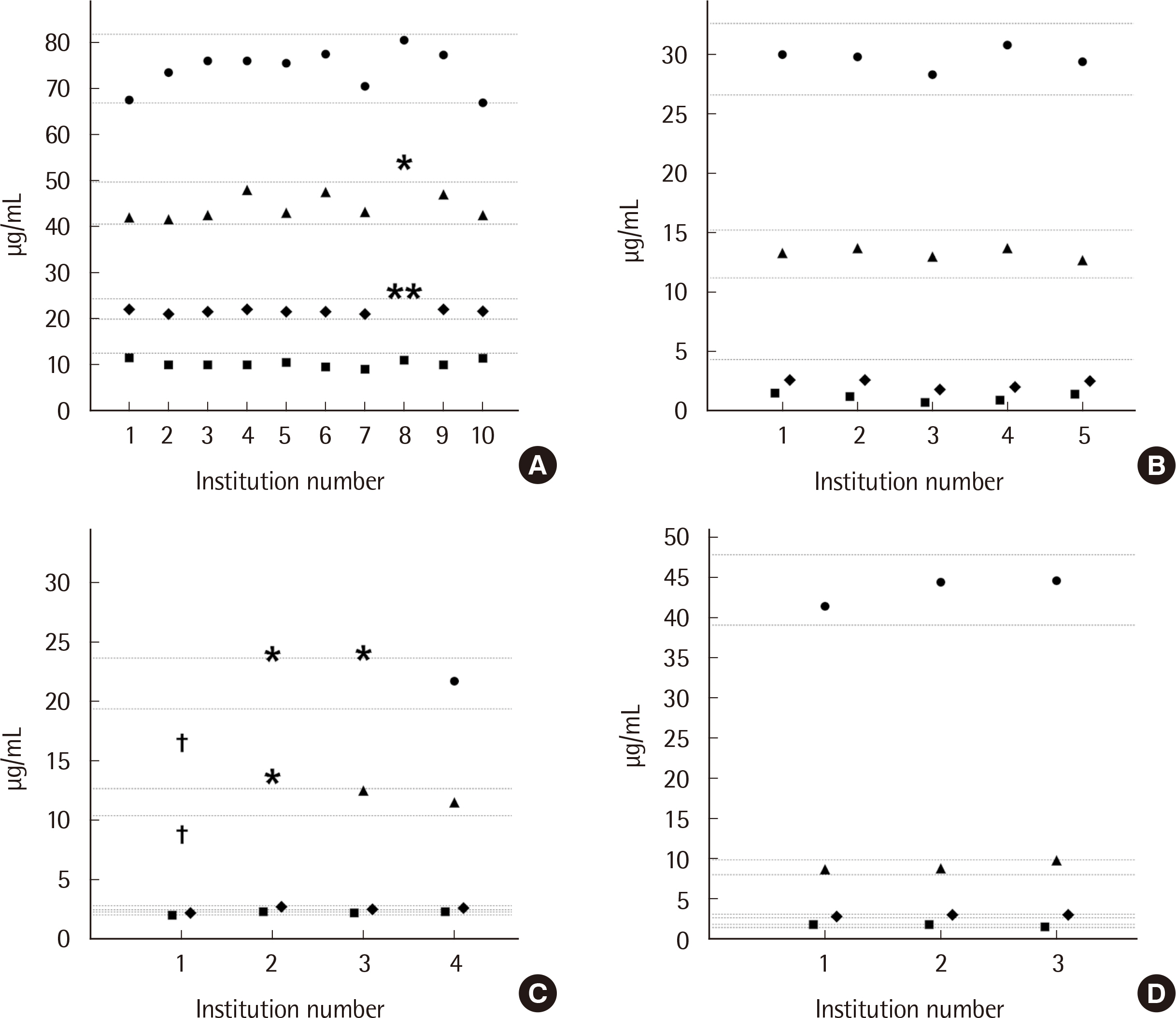
Six instruments from three manufacturers were used. Samples with the lowest drug concentration were reported as below the lower limit of quantitation in most laboratories. Coefficients of variation for within-laboratory values ranged from 1.1% to 10.9% for vancomycin, 0.8% to 18.2% for amikacin, 1.2% to 7.8% for gentamicin, and 1.3% to 6.1% for tobramycin. Based on the overall results of the participants, only one institution’s vancomycin samples standard deviation index exceeded 3, with all other values below 2. The College of American Pathologist criteria were met by all institutions; however, measurement of vancomycin in one laboratory and of gentamycin in three laboratories failed to meet the Royal College of Pathologists of Australasia acceptance criteria.
Conclusions
Although the precision of the antibiotic test in individual institutions was excellent, there was a difference in the measured values between laboratories. Harmonization of antibiotic TDM is needed to reduce inconsistencies in results.
Figure
Reference
-
1. Guo W, Guo GX, Sun C, Zhang J, Rong Z, He J, et al. 2013; Therapeutic drug monitoring of psychotropic drugs in China: a nationwide survey. Ther Drug Monit. 35:816–22. DOI: 10.1097/FTD.0b013e318296a2ff. PMID: 24263641.2. Joerger M, Kraff S, Jaehde U, Hilger RA, Courtney JB, Cline DJ, et al. 2017; Validation of a commercial assay and decision support tool for routine paclitaxel therapeutic drug monitoring (TDM). Ther Drug Monit. 39:617–24. DOI: 10.1097/FTD.0000000000000446. PMID: 28937535.
Article3. Qin X, Rui J, Xia Y, Mu H, Song SH, Raja Aziddin RE, et al. 2018; Multi-center performance evaluations of tacrolimus and cyclosporine electrochemiluminescence immunoassays in the Asia-Pacific region. Ann Lab Med. 38:85–94. DOI: 10.3343/alm.2018.38.2.85. PMID: 29214751. PMCID: PMC5736684.
Article4. Shipkova M, Petrova DT, Rosler AE, Orth M, Engelmayer J, Wieland E, et al. 2014; Comparability and imprecision of 8 frequently used commercially available immunoassays for therapeutic drug monitoring. Ther Drug Monit. 36:433–41. DOI: 10.1097/FTD.0000000000000043. PMID: 24646729.
Article5. Cataldo MA, Tacconelli E, Grilli E, Pea F, Petrosillo N. 2012; Continuous versus intermittent infusion of vancomycin for the treatment of gram-positive infections: systematic review and meta-analysis. J Antimicrob Chemother. 67:17–24. DOI: 10.1093/jac/dkr442. PMID: 22028203.
Article6. Jenkins A, Thomson AH, Brown NM, Semple Y, Sluman C, MacGowan A, et al. 2016; Amikacin use and therapeutic drug monitoring in adults: do dose regimens and drug exposures affect either outcome or adverse events? A systematic review. J Antimicrob Chemother. 71:2754–9. DOI: 10.1093/jac/dkw250. PMID: 27494904.
Article7. Ye ZK, Tang HL, Zhai SD. 2013; Benefits of therapeutic drug monitoring of vancomycin: a systematic review and meta-analysis. PLoS One. 8:e77169. DOI: 10.1371/journal.pone.0077169. PMID: 24204764. PMCID: PMC3799644.
Article8. Streetman DS, Nafziger AN, Destache CJ, Bertino AS Jr. 2001; Individualized pharmacokinetic monitoring results in less aminoglycoside-associated nephrotoxicity and fewer associated costs. Pharmacotherapy. 21:443–51. DOI: 10.1592/phco.21.5.443.34490. PMID: 11310518.
Article9. Revilla N, Martín-Suárez A, Pérez MP, González FM, Fernández de Gatta Mdel M. 2010; Vancomycin dosing assessment in intensive care unit patients based on a population pharmacokinetic/pharmacodynamic simulation. Br J Clin Pharmacol. 70:201–12. DOI: 10.1111/j.1365-2125.2010.03679.x. PMID: 20653673. PMCID: PMC2911550.
Article10. Wilson JF, Davis AC, Tobin CM. 2003; Evaluation of commercial assays for vancomycin and aminoglycosides in serum: a comparison of accuracy and precision based on external quality assessment. J Antimicrob Chemother. 52:78–82. DOI: 10.1093/jac/dkg296. PMID: 12805260.
Article11. Ko DH, Jeong TD, Gu GG, Chun S, Kim JH. 2015; Annual report on the external quality assessment scheme for therapeutic drug monitoring and testing for drugs of abuse in Korea (2014). J Lab Med Qual Assur. 37:12–22. DOI: 10.15263/jlmqa.2015.37.1.12.
Article12. Myers GL, Miller WG. 2016; The international consortium for harmonization of clinical laboratory results (ICHCLR)-A pathway for harmonization. EJIFCC. 27:30–6. PMID: 27683504. PMCID: PMC4975215.13. Royal College of Pathologists of Australasia. 2014. August. Allowable limits of performance. http://www.rcpaqap.com.au/docs/2014/chempath/ALP.pdf. accessed Jun 2019.14. Grubbs FE. 1969; Procedures for detecting outlying observations in samples. Technometrics. 11:1–21. DOI: 10.1080/00401706.1969.10490657.
Article15. Avent ML, Rogers BA, Cheng AC, Paterson DL. 2011; Current use of aminoglycosides: indications, pharmacokinetics and monitoring for toxicity. Intern Med J. 41:441–9. DOI: 10.1111/j.1445-5994.2011.02452.x. PMID: 21309997.
Article16. Ye ZK, Li C, Zhai SD. 2014; Guidelines for therapeutic drug monitoring of vancomycin: a systematic review. PLoS One. 9:e99044. DOI: 10.1371/journal.pone.0099044. PMID: 24932495. PMCID: PMC4059638.
Article17. Martin JH, Norris R, Barras M, Roberts J, Morris R, Doogue M, et al. 2010; Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Clin Biochem Rev. 31:21–4. PMID: 20179794. PMCID: PMC2826264.18. Naito HK, Kwak YS, Hartfiel JL, Park JK, Travers EM, Myers GL, et al. 1993; Matrix effects on proficiency testing materials. Impact on accuracy of cholesterol measurement in laboratories in the nation's largest hospital system. Arch Pathol Lab Med. 117:345–51. PMID: 8466396.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Therapeutic Drug Monitoring (TDM) of Antimicrobial Agents
- Intraventricular Vancomycin Instillation for Postcraniotomy Ventriculitis
- Comparison of Trough-Based and Area Under the Curve-Based Therapeutic Drug Monitoring of Vancomycin: An In Silico Study
- Development of a model to predict vancomycin serum concentration during continuous infusion of vancomycin in critically ill pediatric patients
- Antibacterial Activity of an Antibiotic (K-681) from Streptomyces sp. 681 against Staphylococcus aureus