Int J Stem Cells.
2022 Nov;15(4):347-358. 10.15283/ijsc21250.
Generation of Urothelial Cells from Mouse-Induced Pluripotent Stem Cells
- Affiliations
-
- 1Department of Urology, Yantai Yuhuangding Hospital, Qingdao University, Yantai, China
- 2Department of Urology, The Second Clinical Medical College, Binzhou Medical University, Yantai, China
- KMID: 2536205
- DOI: http://doi.org/10.15283/ijsc21250
Abstract
- Background and Objectives
The search for a suitable alternative for urethral defect is a challenge in the field of urethral tissue engineering. Induced pluripotent stem cells (iPSCs) possess multipotential for differentiation. The in vitro derivation of urothelial cells from mouse-iPSCs (miPSCs) has thus far not been reported. The purpose of this study was to establish an efficient and robust differentiation protocol for the differentiation of miPSCs into urothelial cells.
Methods and Results
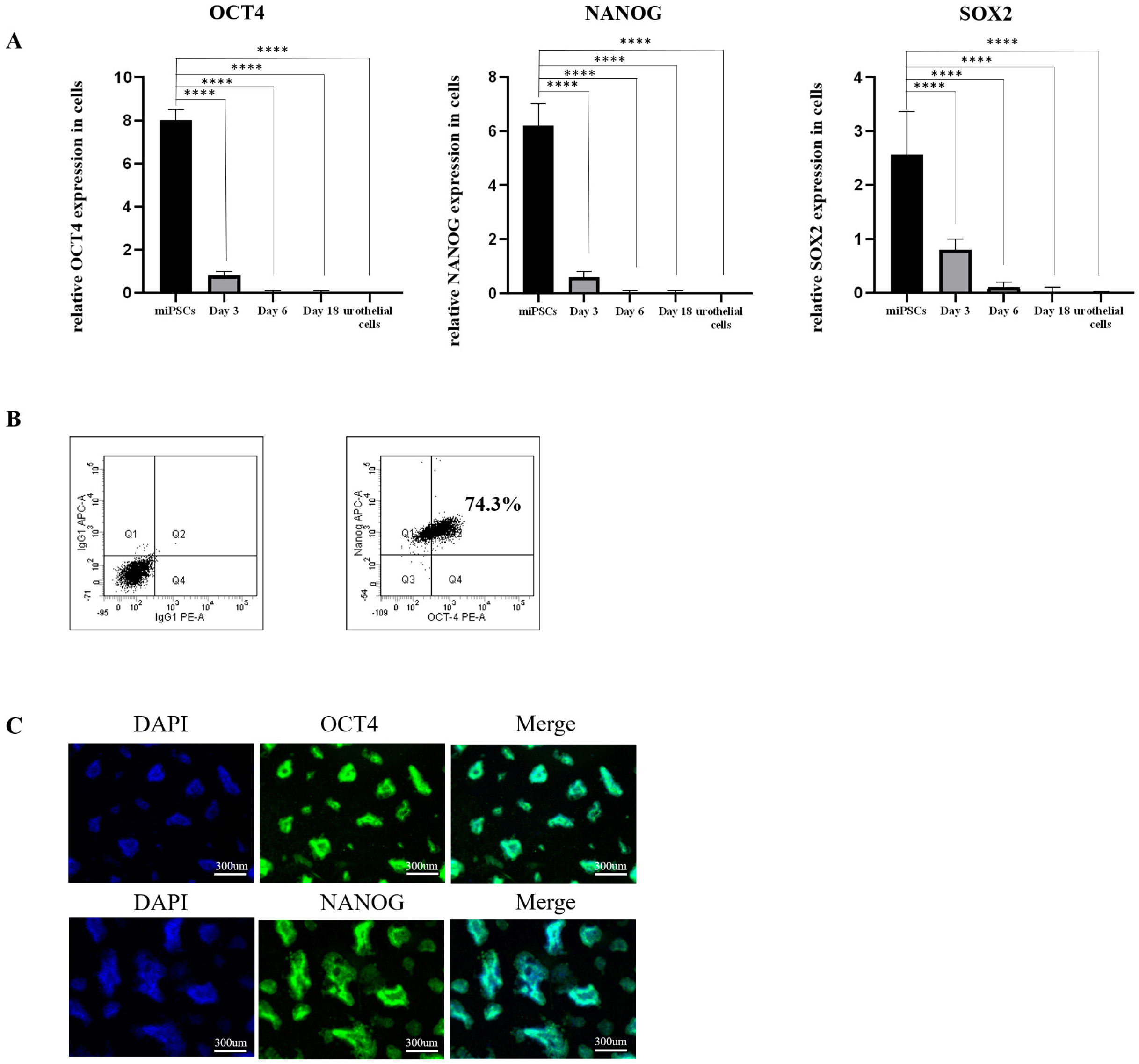
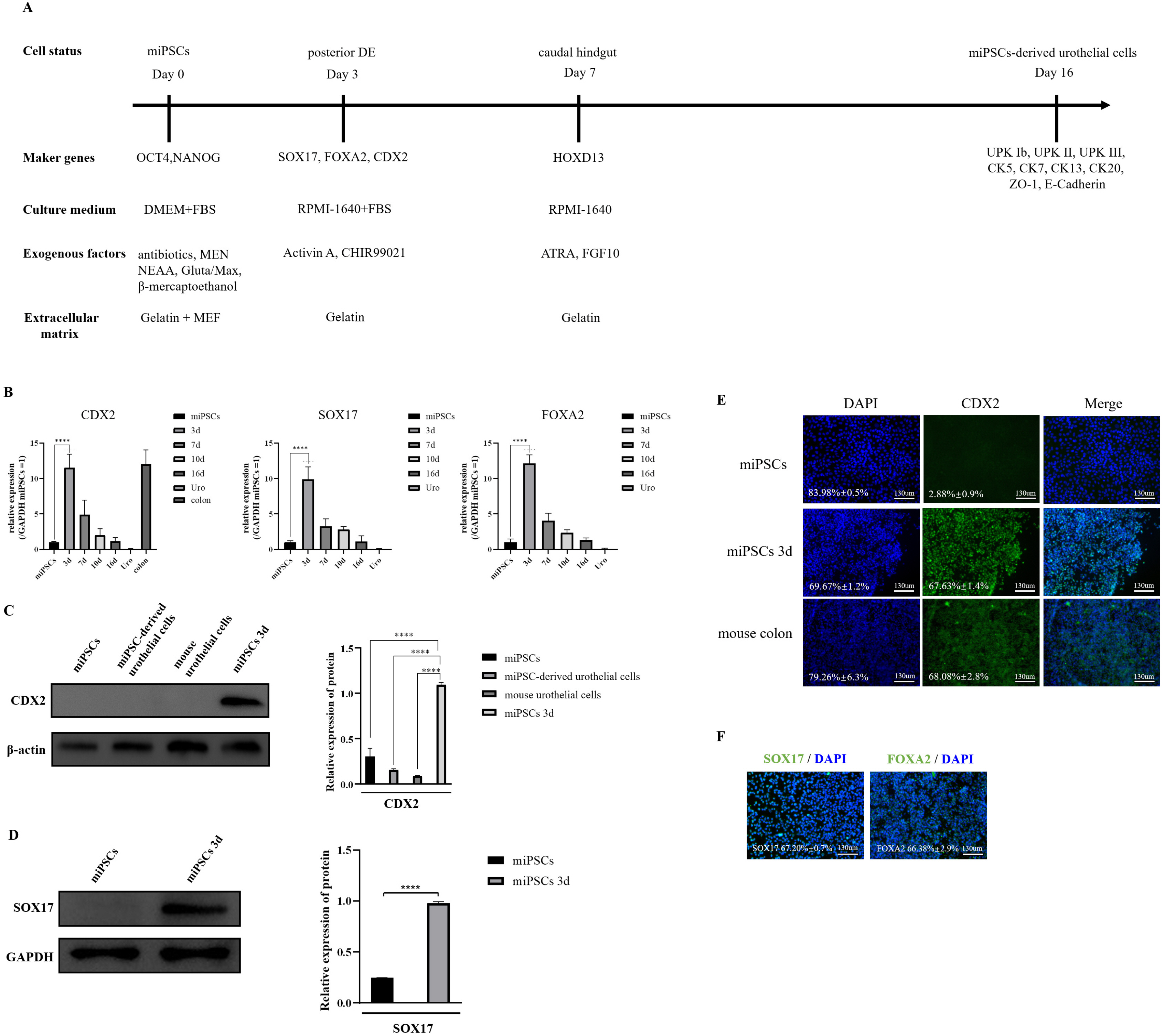
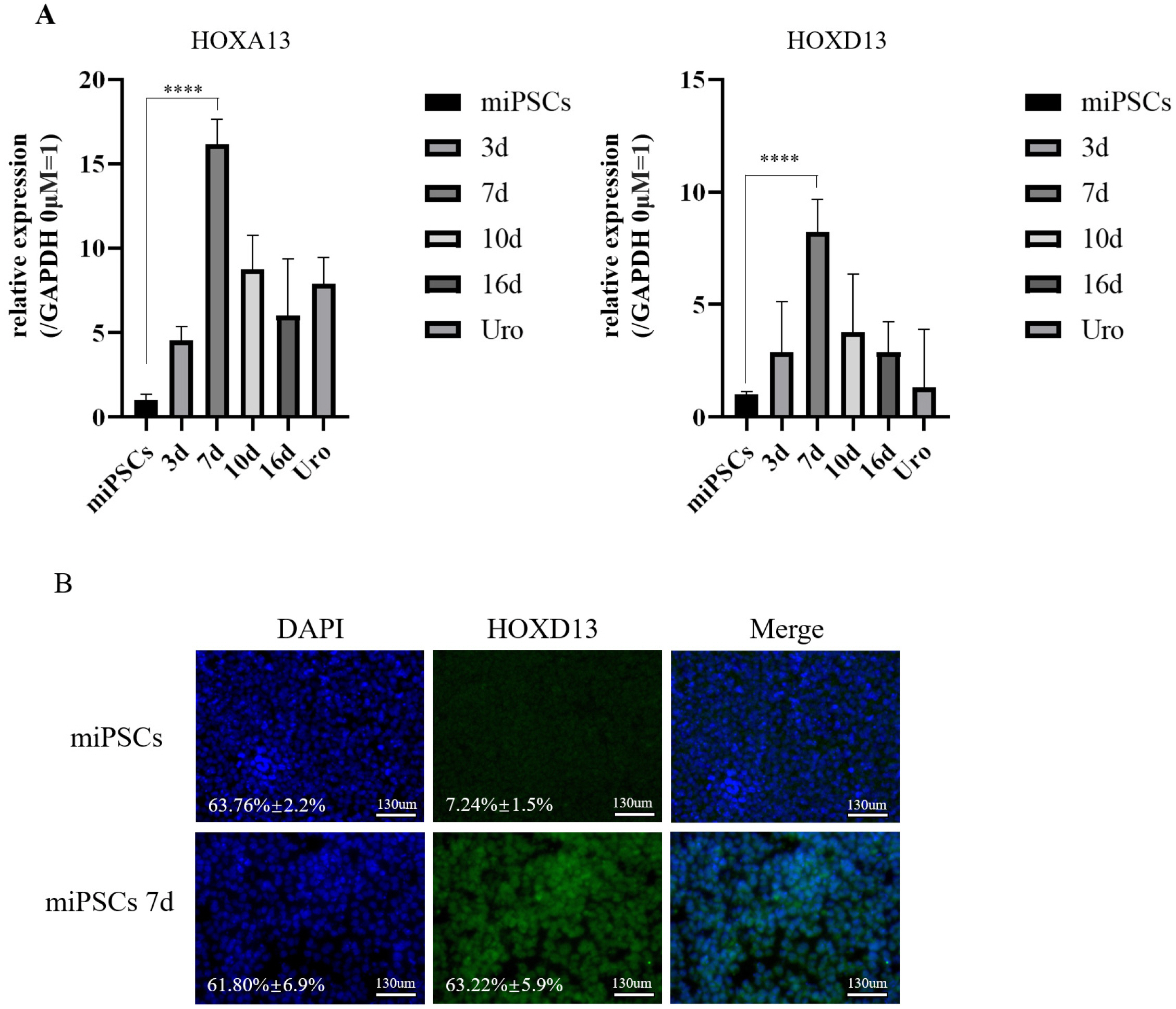
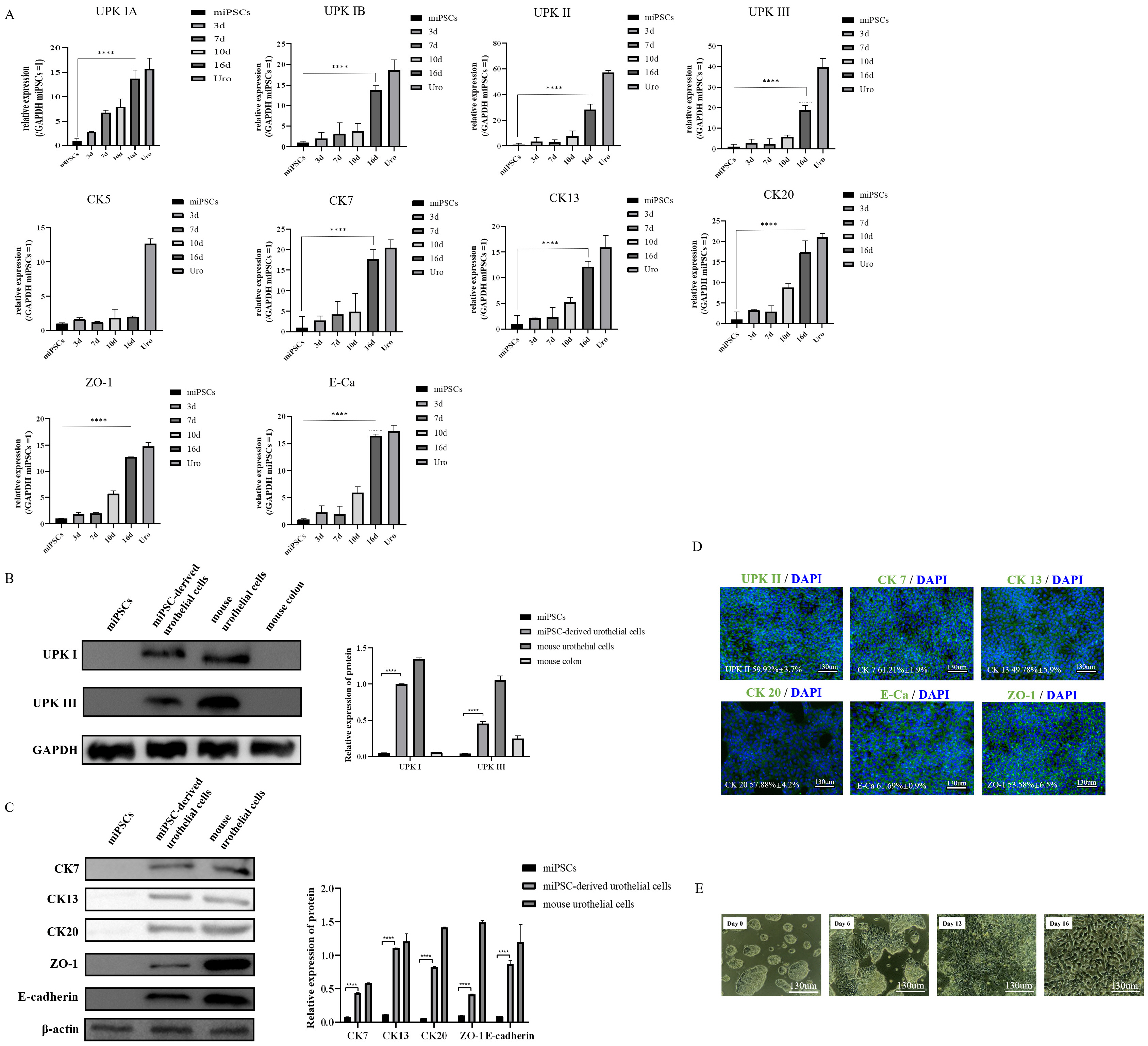
Our protocol made the visualization of differentiation processes of a 2-step approach possible. We firstly induced miPSCs into posterior definitive endoderm (DE) with glycogen synthase kinase-3β (GSK3β) inhibitor and Activin A. We investigated the optimal conditions for DE differentiation with GSK3β inhibitor treatment by varying the treatment time and concentration. Differentiation into urothelial cells, was directed with all-trans retinoic acid (ATRA) and recombinant mouse fibroblast growth factor-10 (FGF-10). Specific markers expressed at each stage of differentiation were validated by flow cytometry, quantitative real-time polymerase chain reaction (qRT-PCR) assay, immunofluorescence staining, and western blotting Assay. The miPSC-derived urothelial cells were successfully in expressed urothelial cell marker genes, proteins, and normal microscopic architecture.
Conclusions
We built a model of directed differentiation of miPSCs into urothelial cells, which may provide the evi-dence for a regenerative potential of miPSCs in preclinical animal studies.
Figure
Reference
-
References
1. Atala A. 2000; Tissue engineering for bladder substitution. World J Urol. 18:364–370. DOI: 10.1007/s003450000152. PMID: 11131316. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=33747672664&origin=inward.
Article2. Kommu SS, Illahi I, Mumtaz F. 2007; Patterns of urethral injury and immediate management. Curr Opin Urol. 17:383–389. DOI: 10.1097/MOU.0b013e3282f0d5fd. PMID: 17921771. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=35148852001&origin=inward.
Article3. Zhang YY, Ludwikowski B, Hurst R, Frey P. 2001; Expansion and long-term culture of differentiated normal rat urothelial cells in vitro. In Vitro Cell Dev Biol Anim. 37:419–429. DOI: 10.1290/1071-2690(2001)037<0419:EALTCO>2.0.CO;2. PMID: 11573816.
Article4. Krajewski W, Piszczek R, Krajewska M, Dembowski J, Zdrojowy R. 2014; Urinary diversion metabolic complications - underestimated problem. Adv Clin Exp Med. 23:633–638. DOI: 10.17219/acem/28251. PMID: 25166450. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84909579005&origin=inward.
Article5. Tanrikut C, McDougal WS. 2004; Acid-base and electrolyte disorders after urinary diversion. World J Urol. 22:168–171. DOI: 10.1007/s00345-004-0430-z. PMID: 15290206. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=16644367674&origin=inward.
Article6. Nieuwenhuijzen JA, de Vries RR, Bex A, van der Poel HG, Meinhardt W, Antonini N, Horenblas S. 2008; Urinary diversions after cystectomy: the association of clinical factors, complications and functional results of four different diversions. Eur Urol. 53:834–842. discussion 842–844. DOI: 10.1016/j.eururo.2007.09.008. PMID: 17904276. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=39449108662&origin=inward.
Article7. Fahmy O, Khairul-Asri MG, Schwentner C, Schubert T, Stenzl A, Zahran MH, Gakis G. 2016; Algorithm for optimal urethral coverage in hypospadias and fistula repair: a systematic review. Eur Urol. 70:293–298. DOI: 10.1016/j.eururo.2015.12.047. PMID: 26776935. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84983164499&origin=inward.
Article8. Erickson BA, Ghareeb GM. 2017; Definition of successful treatment and optimal follow-up after urethral reconstruction for urethral stricture disease. Urol Clin North Am. 44:1–9. DOI: 10.1016/j.ucl.2016.08.001. PMID: 27908363. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84999861278&origin=inward.
Article9. Singh A, Bivalacqua TJ, Sopko N. 2018; Urinary tissue engineering: challenges and opportunities. Sex Med Rev. 6:35–44. DOI: 10.1016/j.sxmr.2017.08.004. PMID: 29066225. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85031814348&origin=inward.
Article10. Galera-Monge T, Zurita-Díaz F, Moreno-Izquierdo A, Fraga MF, Fernández AF, Ayuso C, Garesse R, Gallardo ME. 2016; Generation of a human iPSC line from a patient with an optic atrophy 'plus' phenotype due to a mutation in the OPA1 gene. Stem Cell Res. 16:673–676. DOI: 10.1016/j.scr.2016.03.011. PMID: 27346197. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84962916615&origin=inward.
Article11. Zurita-Díaz F, Galera-Monge T, Moreno-Izquierdo A, Fraga MF, Ayuso C, Fernández AF, Garesse R, Gallardo ME. 2016; Generation of a human iPSC line from a patient with a mitochondrial encephalopathy due to mutations in the GFM1 gene. Stem Cell Res. 16:124–127. DOI: 10.1016/j.scr.2015.12.019. PMID: 27345796. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84952787849&origin=inward.
Article12. Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. 2006; Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 367:1241–1246. DOI: 10.1016/S0140-6736(06)68438-9. PMID: 16631879. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=33646052556&origin=inward.
Article13. Osborn SL, Kurzrock EA. 2015; Production of urothelium from pluripotent stem cells for regenerative applications. Curr Urol Rep. 16:466. DOI: 10.1007/s11934-014-0466-6. PMID: 25404180. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84911360334&origin=inward.
Article14. Chan YY, Sandlin SK, Kurzrock EA, Osborn SL. 2017; The current use of stem cells in bladder tissue regeneration and bioengineering. Biomedicines. 5:4. DOI: 10.3390/biomedicines5010004. PMID: 28536347. PMCID: PMC5423492. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85035350562&origin=inward.
Article15. Suzuki K, Koyanagi-Aoi M, Uehara K, Hinata N, Fujisawa M, Aoi T. 2019; Directed differentiation of human induced pluripotent stem cells into mature stratified bladder urothelium. Sci Rep. 9:10506. DOI: 10.1038/s41598-019-46848-8. PMID: 31324820. PMCID: PMC6642190. PMID: 3ecd9f4f3ceb4aacaeb2a6f812908f88. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85069458585&origin=inward.
Article16. Matsumaru D, Murashima A, Fukushima J, Senda S, Matsushita S, Nakagata N, Miyajima M, Yamada G. 2015; Systematic stereoscopic analyses for cloacal development: the origin of anorectal malformations. Sci Rep. 5:13943. DOI: 10.1038/srep13943. PMID: 26354024. PMCID: PMC4564729. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84941313406&origin=inward.
Article17. Matsuno K, Mae SI, Okada C, Nakamura M, Watanabe A, Toyoda T, Uchida E, Osafune K. 2016; Redefining definitive endoderm subtypes by robust induction of human induced pluripotent stem cells. Differentiation. 92:281–290. DOI: 10.1016/j.diff.2016.04.002. PMID: 27087651.
Article18. Tamminen K, Balboa D, Toivonen S, Pakarinen MP, Wiener Z, Alitalo K, Otonkoski T. 2015; Intestinal commitment and maturation of human pluripotent stem cells is independent of exogenous FGF4 and R-spondin1. PLoS One. 10:e0134551. DOI: 10.1371/journal.pone.0134551. PMID: 26230325. PMCID: PMC4521699. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84941992179&origin=inward.
Article19. Miller AJ, Dye BR, Ferrer-Torres D, Hill DR, Overeem AW, Shea LD, Spence JR. 2019; Generation of lung organoids from human pluripotent stem cells in vitro. Nat Protoc. 14:518–540. DOI: 10.1038/s41596-018-0104-8. PMID: 30664680. PMCID: PMC6531049. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85060351768&origin=inward.
Article20. Oottamasathien S, Wang Y, Williams K, Franco OE, Wills ML, Thomas JC, Saba K, Sharif-Afshar AR, Makari JH, Bhowmick NA, DeMarco RT, Hipkens S, Magnuson M, Brock JW 3rd, Hayward SW, Pope JC 4th, Matusik RJ. 2007; Directed differentiation of embryonic stem cells into bladder tissue. Dev Biol. 304:556–566. DOI: 10.1016/j.ydbio.2007.01.010. PMID: 17289017. PMCID: PMC1994155. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=34047169712&origin=inward.
Article21. Kang L, Wang J, Zhang Y, Kou Z, Gao S. 2009; iPS cells can support full-term development of tetraploid blastocyst-complemented embryos. Cell Stem Cell. 5:135–138. DOI: 10.1016/j.stem.2009.07.001. PMID: 19631602.
Article22. Simara P, Tesarova L, Rehakova D, Farkas S, Salingova B, Kutalkova K, Vavreckova E, Matula P, Matula P, Veverkova L, Koutna I. 2018; Reprogramming of adult peripheral blood cells into human induced pluripotent stem cells as a safe and accessible source of endothelial cells. Stem Cells Dev. 27:10–22. DOI: 10.1089/scd.2017.0132. PMID: 29117787. PMCID: PMC5756468. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85040362601&origin=inward.
Article23. Bar-Nur O, Russ HA, Efrat S, Benvenisty N. 2011; Epigenetic memory and preferential lineage-specific differentiation in induced pluripotent stem cells derived from human pancreatic islet beta cells. Cell Stem Cell. 9:17–23. Erratum in: Cell Stem Cell 2012;11:854. DOI: 10.1016/j.stem.2012.11.007. PMID: 21726830. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84870893668&origin=inward.
Article24. Hu X, Mao C, Fan L, Luo H, Hu Z, Zhang S, Yang Z, Zheng H, Sun H, Fan Y, Yang J, Shi C, Xu Y. 2020; Modeling Parkinson's disease using induced pluripotent stem cells. Stem Cells Int. 2020:1061470. DOI: 10.1155/2020/1061470. PMID: 32256606. PMCID: PMC7091557. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85082678383&origin=inward.
Article25. Hicks RM. 1975; The mammalian urinary bladder: an accommodating organ. Biol Rev Camb Philos Soc. 50:215–246. DOI: 10.1111/j.1469-185X.1975.tb01057.x. PMID: 1100129. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0016799622&origin=inward.26. Acharya P, Beckel J, Ruiz WG, Wang E, Rojas R, Birder L, Apodaca G. 2004; Distribution of the tight junction proteins ZO-1, occludin, and claudin-4, -8, and -12 in bladder epithelium. Am J Physiol Renal Physiol. 287:F305–F318. DOI: 10.1152/ajprenal.00341.2003. PMID: 15068973. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=3242676125&origin=inward.
Article27. Wu XR, Kong XP, Pellicer A, Kreibich G, Sun TT. 2009; Uroplakins in urothelial biology, function, and disease. Kidney Int. 75:1153–1165. DOI: 10.1038/ki.2009.73. PMID: 19340092. PMCID: PMC3717210. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=67349126033&origin=inward.
Article28. McGrath PS, Wells JM. 2015; SnapShot: GI tract development. Cell. 161:176–176.e1. DOI: 10.1016/j.cell.2015.03.014. PMID: 25815994. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84925872121&origin=inward.
Article29. Staack A, Donjacour AA, Brody J, Cunha GR, Carroll P. 2003; Mouse urogenital development: a practical approach. Diffe-rentiation. 71:402–413. DOI: 10.1046/j.1432-0436.2003.7107004.x. PMID: 12969333. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0141888379&origin=inward.
Article30. Gao N, White P, Kaestner KH. 2009; Establishment of intestinal identity and epithelial-mesenchymal signaling by Cdx2. Dev Cell. 16:588–599. DOI: 10.1016/j.devcel.2009.02.010. PMID: 19386267. PMCID: PMC2673200. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=64549114508&origin=inward.
Article31. Kong XT, Deng FM, Hu P, Liang FX, Zhou G, Auerbach AB, Genieser N, Nelson PK, Robbins ES, Shapiro E, Kachar B, Sun TT. 2004; Roles of uroplakins in plaque formation, umbrella cell enlargement, and urinary tract diseases. J Cell Biol. 167:1195–1204. DOI: 10.1083/jcb.200406025. PMID: 15611339. PMCID: PMC2172608. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=19944426308&origin=inward.
Article32. Lobban ED, Smith BA, Hall GD, Harnden P, Roberts P, Selby PJ, Trejdosiewicz LK, Southgate J. 1998; Uroplakin gene expression by normal and neoplastic human urothelium. Am J Pathol. 153:1957–1967. DOI: 10.1016/S0002-9440(10)65709-4. PMID: 9846985. PMCID: PMC1866332. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0031740919&origin=inward.
Article33. Deng FM, Liang FX, Tu L, Resing KA, Hu P, Supino M, Hu CC, Zhou G, Ding M, Kreibich G, Sun TT. 2002; Uroplakin IIIb, a urothelial differentiation marker, dimerizes with uroplakin Ib as an early step of urothelial plaque assembly. J Cell Biol. 159:685–694. DOI: 10.1083/jcb.200204102. PMID: 12446744. PMCID: PMC2173100. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=18744371121&origin=inward.
Article34. Aboushwareb T, Zhou G, Deng FM, Turner C, Andersson KE, Tar M, Zhao W, Melman A, D'Agostino R Jr, Sun TT, Christ GJ. 2009; Alterations in bladder function associated with urothelial defects in uroplakin II and IIIa knockout mice. Neurourol Urodyn. 28:1028–1033. DOI: 10.1002/nau.20688. PMID: 19267388. PMCID: PMC4048927. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=70449379079&origin=inward.
Article35. Rudat C, Grieskamp T, Röhr C, Airik R, Wrede C, Hegermann J, Herrmann BG, Schuster-Gossler K, Kispert A. 2014; Upk3b is dispensable for development and integrity of urothelium and mesothelium. PLoS One. 9:e112112. DOI: 10.1371/journal.pone.0112112. PMID: 25389758. PMCID: PMC4229118. PMID: 1b83fd4de97948f4bc673f43b46c3fff. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84911914615&origin=inward.
Article36. McLin VA, Rankin SA, Zorn AM. 2007; Repression of Wnt/beta-catenin signaling in the anterior endoderm is essential for liver and pancreas development. Development. 134:2207–2217. DOI: 10.1242/dev.001230. PMID: 17507400. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=34347341779&origin=inward.
Article37. Batourina E, Tsai S, Lambert S, Sprenkle P, Viana R, Dutta S, Hensle T, Wang F, Niederreither K, McMahon AP, Carroll TJ, Mendelsohn CL. 2005; Apoptosis induced by vitamin A signaling is crucial for connecting the ureters to the bladder. Nat Genet. 37:1082–1089. DOI: 10.1038/ng1645. PMID: 16186816. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=27144524316&origin=inward.
Article38. Mauney JR, Ramachandran A, Yu RN, Daley GQ, Adam RM, Estrada CR. 2010; All-trans retinoic acid directs urothelial specification of murine embryonic stem cells via GATA4/6 signaling mechanisms. PLoS One. 5:e11513. DOI: 10.1371/journal.pone.0011513. PMID: 20644631. PMCID: PMC2903484. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=77955368268&origin=inward.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Disease-specific pluripotent stem cells
- Induced Pluripotent Stem Cells: Next Generation Stem Cells to Clinical Applications
- Induced pluripotent stem cells and personalized medicine: current progress and future perspectives
- Clinical Applications of Neural Stem Cells for the Treatment of Peripheral Neuropathy
- Questions Surrounding iPS Cells in Japan