Hanyang Med Rev.
2015 Nov;35(4):190-195. 10.7599/hmr.2015.35.4.190.
Induced Pluripotent Stem Cells: Next Generation Stem Cells to Clinical Applications
- Affiliations
-
- 1Department of Surgery, Hanyang University College of Medicine, Seoul, Korea.
- 2Laboratory of Radiation Exposure & Therapeutics, National Radiation Emergency Medical Center, Korea Institute of Radiological & Medical Science, Seoul, Korea. sblee@kirams.re.kr
- KMID: 2129471
- DOI: http://doi.org/10.7599/hmr.2015.35.4.190
Abstract
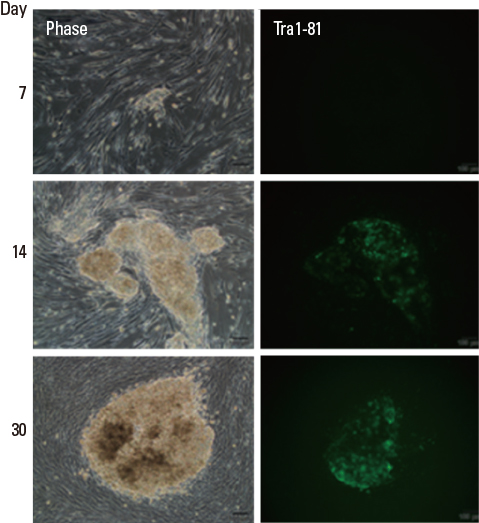
- Induced pluripotent stem cells (iPSC) are specially manipulated cells from somatic cells by the introduction of four factors that are reprogrammed. The properties of iPSC are similar to embryonic stem cells (ESC) characteristic of self-renewal and pluripotency. The technology of reprogramming somatic cells to iPSC enables the generation of patient-specific cells that can be used as powerful tools for drug screening, in vitro models for human disease and autologous transplantation. The iPSC technology provides a priceless resource for regenerative medicine but there are still changing obstacles over the safety of iPSC in avoiding induction of tumorigenicity and maintaining high purity of re-differentiated cells from iPSC to produce more functional cells for cell therapy. A variety of methods to overcome the limitation of iPSC application applied in the clinical setting have been developed. In this review, we summarize the recent progress in iPSC generation and differentiation techniques to facilitate clinical application of iPSC with future potential in regenerative medicine.
MeSH Terms
Figure
Cited by 1 articles
-
New Horizons in Stem Cell Research
Dongho Choi
Hanyang Med Rev. 2015;35(4):187-189. doi: 10.7599/hmr.2015.35.4.187.
Reference
-
1. Keating A. Mesenchymal stromal cells: new directions. Cell Stem Cell. 2012; 10:709–716.
Article2. Svendsen CN. Back to the future: how human induced pluripotent stem cells will transform regenerative medicine. Hum Mol Genet. 2013; 22:R32–R38.
Article3. Chen KG, Mallon BS, McKay RD, Robey PG. Human pluripotent stem cell culture: considerations for maintenance, expansion, and therapeutics. Cell Stem Cell. 2014; 14:13–26.
Article4. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006; 126:663–676.
Article5. Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007; 131:861–872.
Article6. Wakayama S, Jakt ML, Suzuki M, Araki R, Hikichi T, Kishigami S, et al. Equivalency of nuclear transfer-derived embryonic stem cells to those derived from fertilized mouse blastocysts. Stem Cells. 2006; 24:2023–2033.
Article7. Brambrink T, Hochedlinger K, Bell G, Jaenisch R. ES cells derived from cloned and fertilized blastocysts are transcriptionally and functionally indistinguishable. Proc Natl Acad Sci U S A. 2006; 103:933–938.
Article8. Cibelli JB, Stice SL, Golueke PJ, Kane JJ, Jerry J, Blackwell C, et al. Transgenic bovine chimeric offspring produced from somatic cell-derived stem-like cells. Nat Biotechnol. 1998; 16:642–646.
Article9. Wakayama T, Tabar V, Rodriguez I, Perry AC, Studer L, Mombaerts P. Differentiation of embryonic stem cell lines generated from adult somatic cells by nuclear transfer. Science. 2001; 292:740–743.
Article10. Rajamohan D, Matsa E, Kalra S, Crutchley J, Patel A, George V, et al. Current status of drug screening and disease modelling in human pluripotent stem cells. Bioessays. 2013; 35:281–298.
Article11. Halevy T, Urbach A. Comparing ESC and iPSC-Based Models for Human Genetic Disorders. J Clin Med. 2014; 3:1146–1162.
Article12. Ben-David U, Gan QF, Golan-Lev T, Arora P, Yanuka O, Oren YS, et al. Selective elimination of human pluripotent stem cells by an oleate synthesis inhibitor discovered in a high-throughput screen. Cell Stem Cell. 2013; 12:167–179.
Article13. Lee MO, Moon SH, Jeong HC, Yi JY, Lee TH, Shim SH, et al. Inhibition of pluripotent stem cell-derived teratoma formation by small molecules. Proc Natl Acad Sci U S A. 2013; 110:E3281–E3290.
Article14. Sasai Y. Next-generation regenerative medicine: organogenesis from stem cells in 3D culture. Cell Stem Cell. 2013; 12:520–530.
Article15. Ranga A, Gjorevski N, Lutolf MP. Drug discovery through stem cell-based organoid models. Adv Drug Deliv Rev. 2014; 69-70:19–28.
Article16. Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007; 318:1917–1920.
Article17. Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008; 26:101–106.
Article18. Malik N, Rao MS. A review of the methods for human iPSC derivation. Methods Mol Biol. 2013; 997:23–33.
Article19. Higuchi A, Ling QD, Kumar SS, Munusamy MA, Alarfaj AA, Chang Y, et al. Generation of pluripotent stem cells without the use of genetic material. Lab Invest. 2015; 95:26–42.
Article20. Stadtfeld M, Hochedlinger K. Induced pluripotency: history, mechanisms, and applications. Genes Dev. 2010; 24:2239–2263.
Article21. Soldner F, Hockemeyer D, Beard C, Gao Q, Bell GW, Cook EG, et al. Parkinson's disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell. 2009; 136:964–977.
Article22. Sommer CA, Sommer AG, Longmire TA, Christodoulou C, Thomas DD, Gostissa M, et al. Excision of reprogramming transgenes improves the differentiation potential of iPS cells generated with a single excisable vector. Stem Cells. 2010; 28:64–74.
Article23. Zhou W, Freed CR. Adenoviral gene delivery can reprogram human fibroblasts to induced pluripotent stem cells. Stem Cells. 2009; 27:2667–2674.
Article24. Nishimura K, Sano M, Ohtaka M, Furuta B, Umemura Y, Nakajima Y, et al. Development ofdefective and persistent Sendai virus vector: a unique gene delivery/expression system ideal for cell reprogramming. J Biol Chem. 2011; 286:4760–4771.25. Zhou H, Wu S, Joo JY, Zhu S, Han DW, Lin T, et al. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell. 2009; 4:381–384.
Article26. Warren L, Manos PD, Ahfeldt T, Loh YH, Li H, Lau F, et al. Highly efficient reprogrammingto pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010; 7:618–630.
Article27. Anokye-Danso F, Trivedi CM, Juhr D, Gupta M, Cui Z, Tian Y, et al. Highly efficient miRNA-mediated reprogramming of mouse and human somatic cells to pluripotency. Cell Stem Cell. 2011; 8:376–388.
Article28. Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin II, et al. Human induced pluripotentstem cells free of vector and transgene sequences. Science. 2009; 324:797–801.
Article29. Orkin SH, Hochedlinger K. Chromatin connections to pluripotency and cellular reprogramming. Cell. 2011; 145:835–850.
Article30. Liang G, Zhang Y. Embryonic stem cell and induced pluripotent stem cell: an epigenetic perspective. Cell Res. 2013; 23:49–69.
Article31. Huangfu D, Maehr R, Guo W, Eijkelenboom A, Snitow M, Chen AE, et al. Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nat Biotechnol. 2008; 26:795–797.
Article32. Huangfu D, Osafune K, Maehr R, Guo W, Eijkelenboom A, Chen S, et al. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotechnol. 2008; 26:1269–1275.
Article33. Mali P, Chou BK, Yen J, Ye Z, Zou J, Dowey S, et al. Butyrate greatly enhances derivation of human induced pluripotent stem cells by promoting epigenetic remodeling and the expression of pluripotency-associated genes. Stem Cells. 2010; 28:713–720.
Article34. Hou P, Li Y, Zhang X, Liu C, Guan J, Li H, et al. Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science. 2013; 341:651–654.
Article35. Lowry WE, Richter L, Yachechko R, Pyle AD, Tchieu J, Sridharan R, et al. Generation of human induced pluripotent stem cells from dermal fibroblasts. Proc Natl Acad Sci U S A. 2008; 105:2883–2888.
Article36. Lee SB, Seo D, Choi D, Park KY, Holczbauer A, Marquardt JU, et al. Contribution of hepatic lineage stage-specific donor memory to the differential potential of induced mouse pluripotent stem cells. Stem Cells. 2012; 30:997–1007.
Article37. Acimovic I, Vilotic A, Pesl M, Lacampagne A, Dvorak P, Rotrekl V, et al. Human pluripotent stem cell-derived cardiomyocytes as research and therapeutic tools. BioMed Res Int. 2014; 2014:512831.
Article38. Kim EM, Manzar G, Zavazava N. Human iPS cell-derived hematopoietic progenitor cells induce T-cell anergy in in vitro-generated alloreactive CD8(+) T cells. Blood. 2013; 121:5167–5175.
Article39. Schondorf DC, Aureli M, McAllister FE, Hindley CJ, Mayer F, Schmid B, et al. iPSC-derived neurons from GBA1-associated Parkinson's disease patients show autophagic defects and impaired calcium homeostasis. Nat Commun. 2014; 5:4028.
Article40. Sun C, Wilson GS, Fan JG, Qiao L. Potential applications of induced pluripotent stem cells (iPSCs) in hepatology research. Curr Stem Cell Res Ther. 2015; 10:208–215.
Article41. Ye Z, Chou BK, Cheng L. Promise and challenges of human iPSC-based hematologic disease modeling and treatment. Int J Hematol. 2012; 95:601–609.
Article42. Moledina F, Clarke G, Oskooei A, Onishi K, Gunther A, Zandstra PW. Predictive microfluidic control of regulatory ligand trajectories in individual pluripotent cells. Proc Natl Acad Sci U S A. 2012; 109:3264–3269.
Article43. Lock LT, Tzanakakis ES. Expansion and differentiation of human embryonic stem cells to endoderm progeny in a microcarrier stirred-suspension culture. Tissue Eng Part A. 2009; 15:2051–2063.
Article44. DeQuach JA, Mezzano V, Miglani A, Lange S, Keller GM, Sheikh F, et al. Simple and high yielding method for preparing tissue specific extracellular matrix coatings for cell culture. PLoS One. 2010; 5:e13039.
Article45. Eiraku M, Takata N, Ishibashi H, Kawada M, Sakakura E, Okuda S, et al. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature. 2011; 472:51–56.
Article46. Efthymiou A, Shaltouki A, Steiner JP, Jha B, Heman-Ackah SM, Swistowski A, et al. Functional screening assays with neurons generated from pluripotent stem cell-derived neural stem cells. J Biomol Screen. 2014; 19:32–43.
Article47. Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011; 470:105–109.
Article48. Takebe T, Sekine K, Enomura M, Koike H, Kimura M, Ogaeri T, et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013; 499:481–484.
Article49. Willenbring H, Soto-Gutierrez A. Transplantable liver organoids made from only three ingredients. Cell Stem Cell. 2013; 13:139–140.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Applications of Neural Stem Cells for the Treatment of Peripheral Neuropathy
- Disease-specific pluripotent stem cells
- Induced pluripotent stem cells and personalized medicine: current progress and future perspectives
- Questions Surrounding iPS Cells in Japan
- Vascularized Organoids: A More Complete Model