Diabetes Metab J.
2022 Nov;46(6):948-952. 10.4093/dmj.2021.0332.
Glutamic Acid Decarboxylase and Tyrosine Phosphatase-Related Islet Antigen-2 Positivity among Children and Adolescents with Diabetes in Korea
- Affiliations
-
- 1Department of Pediatrics, Seoul National University Children’s Hospital, Seoul, Korea
- 2Department of Pediatrics, Seoul National University Bundang Hospital, Seongnam, Korea
- 3Department of Pediatrics, Seoul National University College of Medicine, Seoul, Korea
- 4Department of Pediatrics, Seoul Metropolitan Government Seoul National University Boramae Medical Center, Seoul, Korea
- KMID: 2536153
- DOI: http://doi.org/10.4093/dmj.2021.0332
Abstract
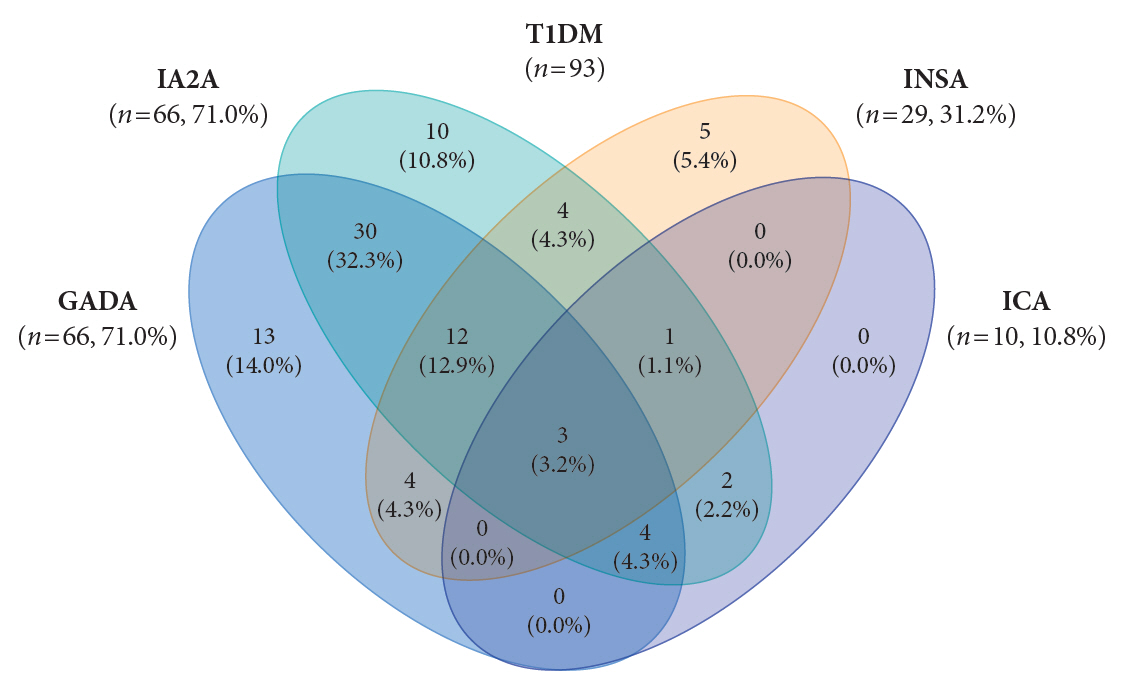
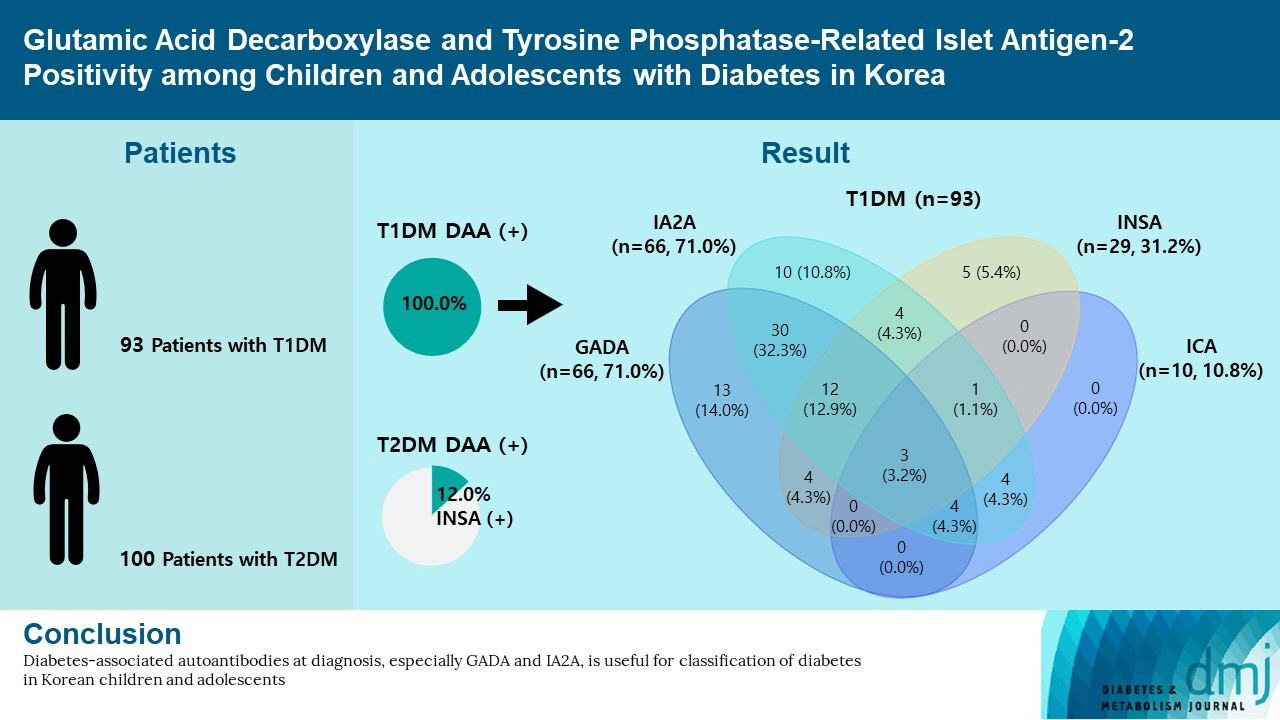
- Autoantibodies against glutamic acid decarboxylase (GADA), tyrosine phosphatase-related islet antigen 2 (IA2A), insulin (INSA), and islet cells (ICA) are critical for determining the type of diabetes and management strategy in new-onset diabetes mellitus (NODM), but there have been few reports of all diabetes-associated autoantibody (DAA) in Korea. We retrospectively analyzed 193 patients with NODM aged 0 to 18 years who were followed at two tertiary centers in Korea (2017 to 2021). Patients with type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM) were 93 (48.2%) and 100 (51.8%), respectively. In T1DM patients, the DAA positivity rate was 94.6%; prevalence of GADA, IA2A, INSA, and ICA was 71.0%, 71.0%, 31.2%, and 10.8%, respectively; and IA2A added 10.7% point autoantibody positivity (83.9% for GADA+INSA+ICA and 94.6% for GADA+INSA+ICA+IA2A). Among the patients with T2DM, 12 (12.0%) were positive for DAA, and all were positive for INSA. These findings suggest that DAA at diagnosis, especially GADA and IA2A, is useful for classifying diabetes in Korean children and adolescents.
Figure
Cited by 2 articles
-
Diagnostic and Therapeutic Strategies of Type 2 Diabetes Mellitus in Youth
Hwa Young Kim, Jae Hyun Kim
Ewha Med J. 2022;45(3):e3. doi: 10.12771/emj.2022.e3.Immune-Checkpoint Inhibitors-Induced Type 1 Diabetes Mellitus: From Its Molecular Mechanisms to Clinical Practice
Yun Kyung Cho, Chang Hee Jung
Diabetes Metab J. 2023;47(6):757-766. doi: 10.4093/dmj.2023.0072.
Reference
-
1. Katsarou A, Gudbjornsdottir S, Rawshani A, Dabelea D, Bonifacio E, Anderson BJ, et al. Type 1 diabetes mellitus. Nat Rev Dis Primers. 2017; 3:17016.
Article2. Zeitler P, Arslanian S, Fu J, Pinhas-Hamiel O, Reinehr T, Tandon N, et al. ISPAD clinical practice consensus guidelines 2018: type 2 diabetes mellitus in youth. Pediatr Diabetes. 2018; 19 Suppl 27:28–46.
Article3. Dayan CM, Besser R, Oram RA, Hagopian W, Vatish M, Bendor-Samuel O, et al. Preventing type 1 diabetes in childhood. Science. 2021; 373:506–10.
Article4. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2021. Diabetes Care. 2021; 44(Suppl 1):S15–33.5. Nieto J, Castillo B, Astudillo M, Tosur M, Balasubramanyam A, Pietropaolo M, et al. Islet autoantibody types mark differential clinical characteristics at diagnosis of pediatric type 1 diabetes. Pediatr Diabetes. 2021; 22:882–8.
Article6. Wenzlau JM, Hutton JC. Novel diabetes autoantibodies and prediction of type 1 diabetes. Curr Diab Rep. 2013; 13:608–15.
Article7. Ushijima K, Okuno M, Ayabe T, Kikuchi N, Kawamura T, Urakami T, et al. Low prevalence of maternal microchimerism in peripheral blood of Japanese children with type 1 diabetes. Diabet Med. 2020; 37:2131–5.
Article8. Hou L, Li X, Liu L, Wei H, Xiong F, Du H, et al. A multicenter survey of type I diabetes mellitus in Chinese children. Front Endocrinol (Lausanne). 2021; 12:583114.
Article9. Vipin VP, Zaidi G, Watson K, Colman PG, Prakash S, Agrawal S, et al. High prevalence of idiopathic (islet antibody-negative) type 1 diabetes among Indian children and adolescents. Pediatr Diabetes. 2021; 22:47–51.
Article10. Choi JH, Kim MS, Kim CJ, Kim JD, Lee DY. Comparison of clinical and laboratory characteristics in children with type 1 diabetes according to pancreatic autoantibodies. Korean J Pediatr. 2010; 53:414–9.
Article11. Park Y, Lee H, Takino H, Abiru N, Kawasaki E, Eisenbarth GS. Evaluation of the efficacy of the combination of multiple autoantibodies to islet-specific antigens in Korean type 1 diabetic patients. Acta Diabetol. 2001; 38:51–6.12. Gao X, Sun W, Wang Y, Zhang Y, Li R, Huang J, et al. Prevalence of positive islet autoantibody in type 2 diabetes patients: a cross-sectional study in a Chinese community. Endocr Connect. 2019; 8:1493–502.
Article13. Kim JH, Yun S, Hwang SS, Shim JO, Chae HW, Lee YJ, et al. The 2017 Korean National Growth Charts for children and adolescents: development, improvement, and prospects. Korean J Pediatr. 2018; 61:135–49.
Article14. Betterle C, Fusari A, Presotto F, Dal Pra C, Pedini B, Lazzarotto F, et al. Pancreatic autoantibodies in Italian patients with newly diagnosed type 1 diabetes mellitus under the age of 20 years. Ann N Y Acad Sci. 2002; 958:271–5.
Article15. Pilla SJ, Balasubramanyam A, Knowler WC, Lazo M, Nathan DM, Pi-Sunyer X, et al. Islet autoantibody positivity in overweight and obese adults with type 2 diabetes. Autoimmunity. 2018; 51:408–16.
Article16. Ong YH, Koh W, Ng ML, Tam ZY, Lim SC, Boehm BO, et al. Glutamic acid decarboxylase and islet antigen 2 antibody profiles in people with adult-onset diabetes mellitus: a comparison between mixed ethnic populations in Singapore and Germany. Diabet Med. 2017; 34:1145–53.
Article17. Ji MC, Chae HW, Kim HS, Kim DH. The clinical characteristics of type 2 diabetes mellitus with and without beta-cell autoantibody in children and adolescents. J Korean Soc Pediatr Endocrinol. 2010; 15:93–9.18. Pihoker C, Gilliam LK, Hampe CS, Lernmark A. Autoantibodies in diabetes. Diabetes. 2005; 54 Suppl 2:S52–61.
Article19. Maedler K, Sergeev P, Ris F, Oberholzer J, Joller-Jemelka HI, Spinas GA, et al. Glucose-induced β cell production of IL-1β contributes to glucotoxicity in human pancreatic islets. J Clin Invest. 2017; 127:1589.
Article20. Rochmah N, Faizi M, Windarti SW. Zinc transporter 8 autoantibody in the diagnosis of type 1 diabetes in children. Clin Exp Pediatr. 2020; 63:402–5.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Analysis of the Persistence of Islet Cell Cytoplasmic Antibodies and Glutamic Acid Decarboxylase ( GAD ) 65 Antibodies in Type 1 Diabetic Children
- Glutamic acid decarboxylase(gad):an autoantigen in insulin-dependent diabetes mellitus
- Thyroid autoimmunity in children and adolescents with newly diagnosed type 1 diabetes mellitus
- Comparison of the prevalence of islet autoantibodies according to age and disease duration in patients with type 1 diabetes mellitus
- The effects of electroconvulsive shock on glutamate decarboxylase and glutamine synthetase activity in adrenalectomized rat hippocampus