Korean J Physiol Pharmacol.
2022 Nov;26(6):541-556. 10.4196/kjpp.2022.26.6.541.
Gaseous signal molecule SO2 regulates autophagy through PI3K/ AKT pathway inhibits cardiomyocyte apoptosis and improves myocardial fibrosis in rats with type II diabetes
- Affiliations
-
- 1Department of Pharmacy, The Second Affiliated Hospital, Hengyang Medical School, University of South China, Hengyang 421000, China
- 2Department of Cardiology, The First Affiliated Hospital, Hengyang Medical School, University of South China, Hengyang 421000, China
- 3Department of General Practice, The Second Affiliated Hospital, Hengyang Medical School, University of South China, Hengyang 421000, China
- 4School of Pharmaceutical Science of University of South China, Hengyang 421000, China
- 5Department of Ultrasound Medicine, The First Affiliated Hospital, Hengyang Medical School, University of South China, Hengyang 421000, China
- 6Department of Critical Care Medicine, The Affiliated Nanhua Hospital, Hengyang Medical School, University of South China, Hengyang 421000, China
- 7Department of Cardiology, The Affiliated Nanhua Hospital, Hengyang Medical School, University of South China, Hengyang 421000, China
- 8Department of Cardiology, Shenzhen Longhua District Central Hospital, Longhua Central Hospital Affiliated Guang-dong Medical University, Shenzhen 518000, China
- KMID: 2534529
- DOI: http://doi.org/10.4196/kjpp.2022.26.6.541
Abstract
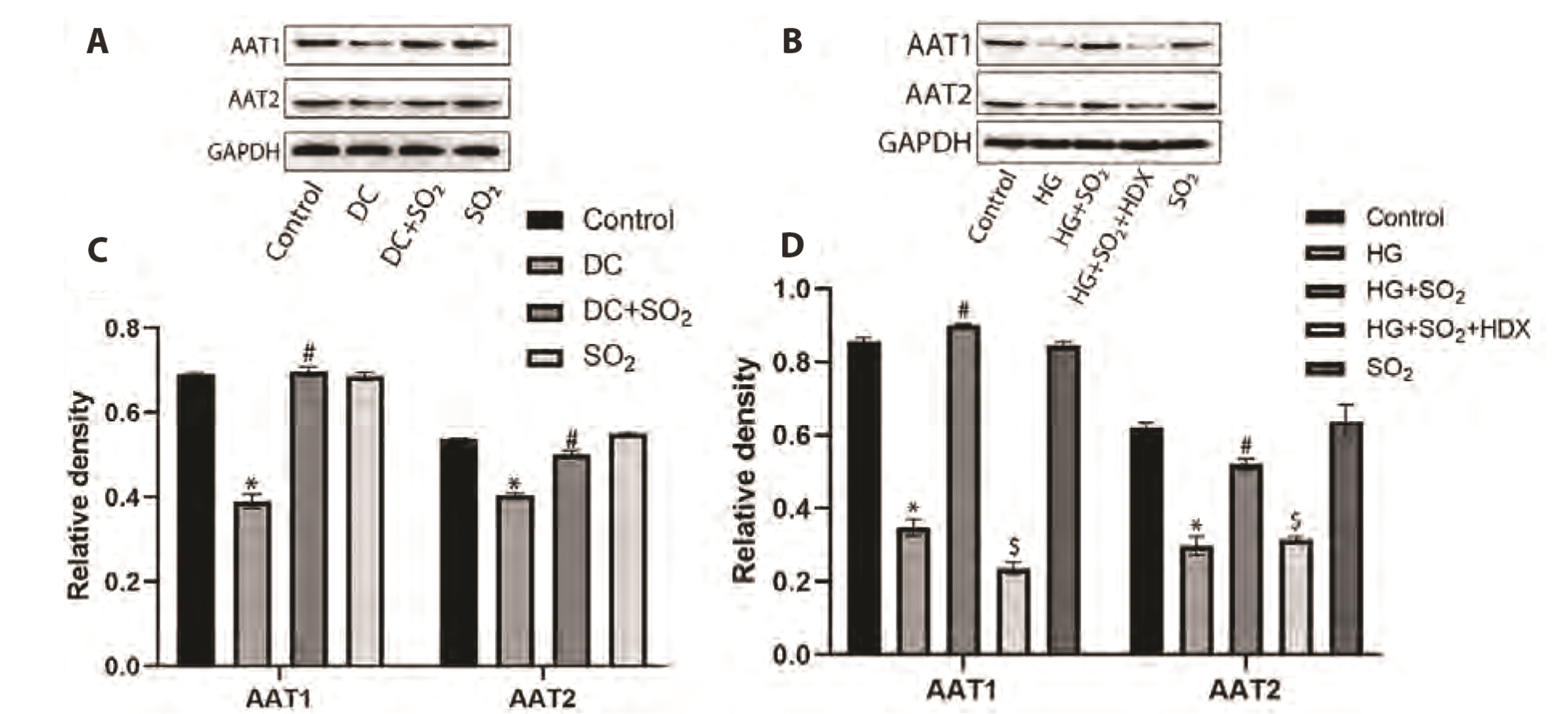
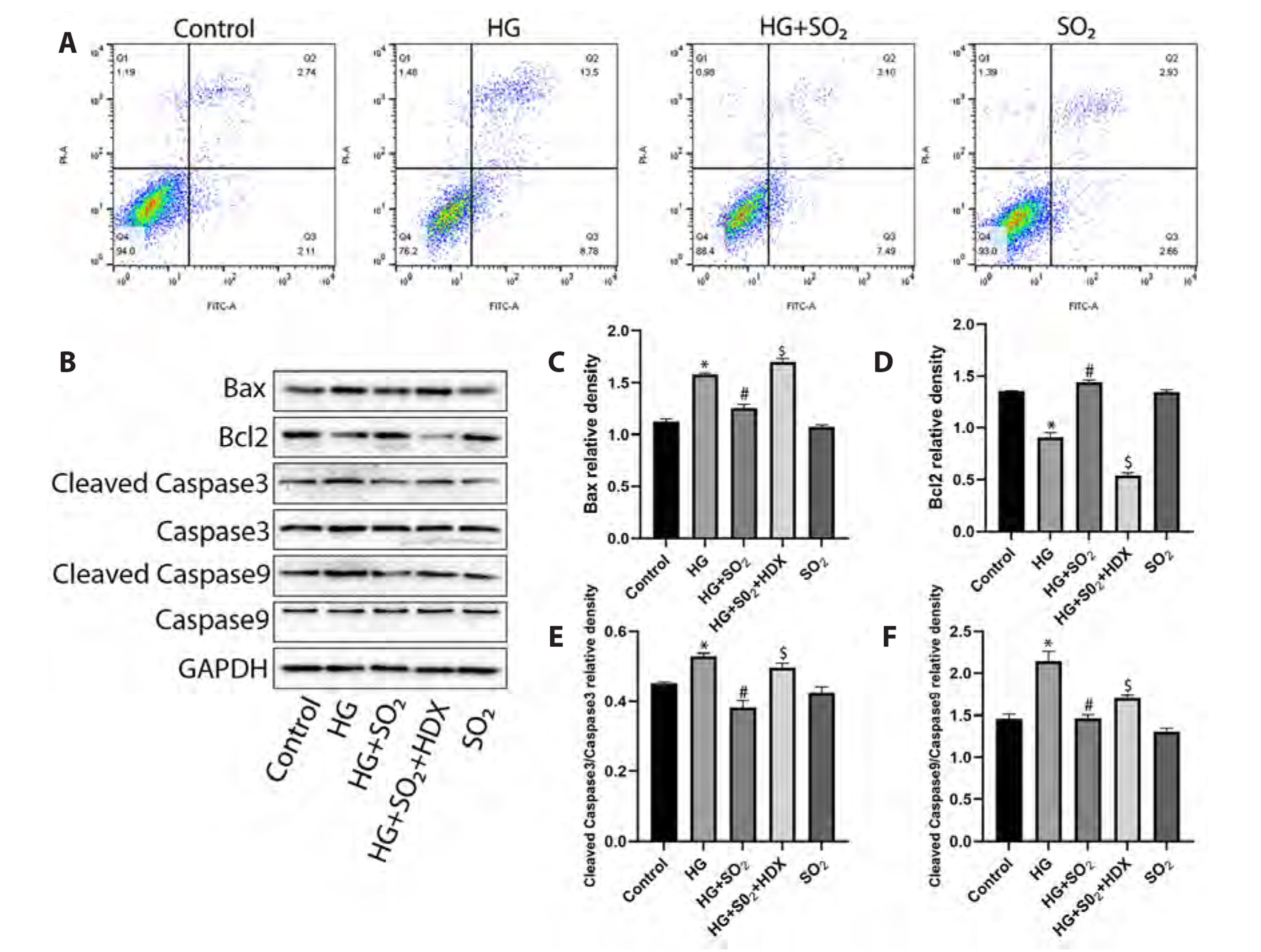
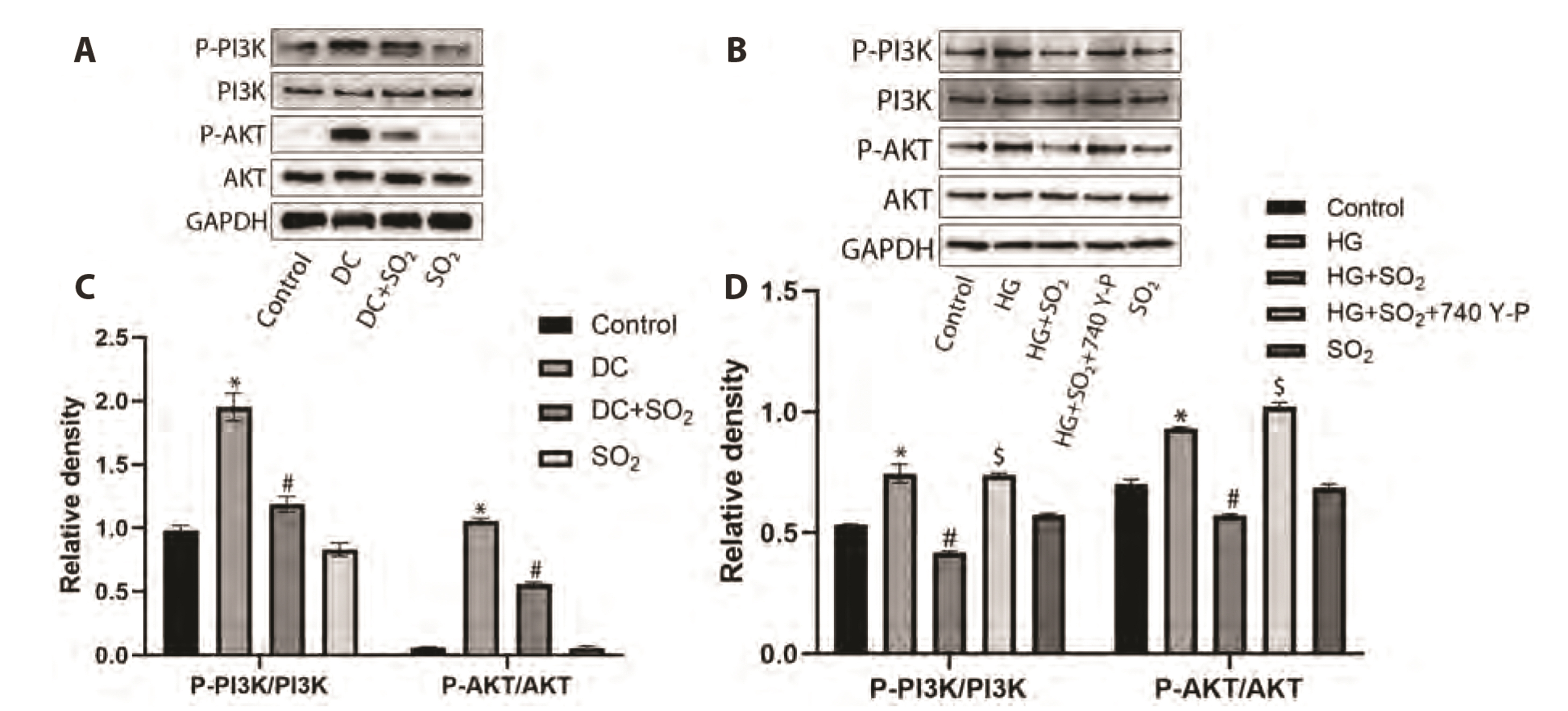
- Myocardial fibrosis is a key link in the occurrence and development of diabetic cardiomyopathy. Its etiology is complex, and the effect of drugs is not good. Cardiomyocyte apoptosis is an important cause of myocardial fibrosis. The purpose of this study was to investigate the effect of gaseous signal molecule sulfur dioxide (SO2 ) on diabetic myocardial fibrosis and its internal regulatory mechanism. Masson and TUNEL staining, Western-blot, transmission electron microscopy, RT-qPCR, immunofluorescence staining, and flow cytometry were used in the study, and the interstitial collagen deposition, autophagy, apoptosis, and changes in phosphatidylinositol 3-kinase (PI3K)/AKT pathways were evaluated from in vivo and in vitro experiments. The results showed that diabetic myocardial fibrosis was accompanied by cardiomyocyte apoptosis and down-regulation of endogenous SO2 -producing enzyme aspartate aminotransferase (AAT)1/2 . However, exogenous SO2 donors could up-regulate AAT1/2 , reduce apoptosis of cardiomyocytes induced by diabetic rats or high glucose, inhibit phosphorylation of PI3K/AKT protein, up-regulate autophagy, and reduce interstitial collagen deposition. In conclusion, the results of this study suggest that the gaseous signal molecule SO2 can inhibit the PI3K/AKT pathway to promote cytoprotective autophagy and inhibit cardiomyocyte apoptosis to improve myocardial fibrosis in diabetic rats. The results of this study are expected to provide new targets and intervention strategies for the prevention and treatment of diabetic cardiomyopathy.
Figure
Cited by 1 articles
-
Experimental model and novel therapeutic targets for non-alcoholic fatty liver disease development
Yujin Jin, Kyung-Sun Heo
Korean J Physiol Pharmacol. 2023;27(4):299-310. doi: 10.4196/kjpp.2023.27.4.299.
Reference
-
1. Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, Shaw JE, Bright D, Williams R. 2019; Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 157:107843. DOI: 10.1016/j.diabres.2019.107843. PMID: 31518657. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85075731827&origin=inward.
Article2. Horton WB, Barrett EJ. 2021; Microvascular dysfunction in diabetes mellitus and cardiometabolic disease. Endocr Rev. 42:29–55. DOI: 10.1210/endrev/bnaa025. PMID: 33125468. PMCID: PMC7846151. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85100737841&origin=inward.
Article3. Dall TM, Yang W, Gillespie K, Mocarski M, Byrne E, Cintina I, Beronja K, Semilla AP, Iacobucci W, Hogan PF. 2019; The economic burden of elevated blood glucose levels in 2017: diagnosed and undiagnosed diabetes, gestational diabetes mellitus, and prediabetes. Diabetes Care. 42:1661–1668. DOI: 10.2337/dc18-1226. PMID: 30940641. PMCID: PMC6702607. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85071497405&origin=inward.
Article4. Lam HCY, Chan JCN, Luk AOY, Chan EYY, Goggins WB. 2018; Short-term association between ambient temperature and acute myocardial infarction hospitalizations for diabetes mellitus patients: a time series study. PLoS Med. 15:e1002612. DOI: 10.1371/journal.pmed.1002612. PMID: 30016318. PMCID: PMC6049878. PMID: c202e480e9084ab98d81b89a10223921. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85051114509&origin=inward.
Article5. Seferović PM, Polovina M, Bauersachs J, Arad M, Ben Gal T, Lund LH, Felix SB, Arbustini E, Caforio ALP, Farmakis D, Filippatos GS, Gialafos E, Kanjuh V, Krljanac G, Limongelli G, Linhart A, Lyon AR, Maksimović R, Miličić D, Milinković I, et al. 2019; Heart failure in cardiomyopathies: a position paper from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 21:553–576. DOI: 10.1002/ejhf.1461. PMID: 30989768. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85064571312&origin=inward.
Article6. Tromp J, Lim SL, Tay WT, Teng TK, Chandramouli C, Ouwerkerk W, Wander GS, Sawhney JPS, Yap J, MacDonald MR, Ling LH, Sattar N, McMurray JJV, Richards AM, Anand I, Lam CSP. 2019; Microvascular disease in patients with diabetes with heart failure and reduced ejection versus preserved ejection fraction. Diabetes Care. 42:1792–1799. DOI: 10.2337/dc18-2515. PMID: 31292141. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85071615771&origin=inward.
Article7. Jia G, Hill MA, Sowers JR. 2018; Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ Res. 122:624–638. DOI: 10.1161/CIRCRESAHA.117.311586. PMID: 29449364. PMCID: PMC5819359. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85044730815&origin=inward.8. Kenny HC, Abel ED. 2019; Heart failure in type 2 diabetes mellitus. Circ Res. 124:121–141. DOI: 10.1161/CIRCRESAHA.118.311371. PMID: 30605420. PMCID: PMC6447311. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85059500922&origin=inward.
Article9. Seferović PM, Petrie MC, Filippatos GS, Anker SD, Rosano G, Bauersachs J, Paulus WJ, Komajda M, Cosentino F, de Boer RA, Farmakis D, Doehner W, Lambrinou E, Lopatin Y, Piepoli MF, Theodorakis MJ, Wiggers H, Lekakis J, Mebazaa A, Mamas MA, et al. 2018; Type 2 diabetes mellitus and heart failure: a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 20:853–872. DOI: 10.1002/ejhf.1170. PMID: 29520964. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85046398177&origin=inward.
Article10. Liu M, López de Juan Abad B, Cheng K. 2021; Cardiac fibrosis: myofibroblast-mediated pathological regulation and drug delivery strategies. Adv Drug Deliv Rev. 173:504–519. DOI: 10.1016/j.addr.2021.03.021. PMID: 33831476. PMCID: PMC8299409. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85107696062&origin=inward.11. Miki T, Yuda S, Kouzu H, Miura T. 2013; Diabetic cardiomyopathy: pathophysiology and clinical features. Heart Fail Rev. 18:149–166. DOI: 10.1007/s10741-012-9313-3. PMID: 22453289. PMCID: PMC3593009. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84876569165&origin=inward.
Article12. Ma T, Huang X, Zheng H, Huang G, Li W, Liu X, Liang J, Cao Y, Hu Y, Huang Y. 2021; SFRP2 improves mitochondrial dynamics and mitochondrial biogenesis, oxidative stress, and apoptosis in diabetic cardiomyopathy. Oxid Med Cell Longev. 2021:9265016. DOI: 10.1155/2021/9265016. PMID: 34790288. PMCID: PMC8592716. PMID: 4f884f465e5f482482abd57bf85ffaa8. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85119959525&origin=inward.
Article13. Yu W, Zha W, Ren J. 2018; Exendin-4 and liraglutide attenuate glucose toxicity-induced cardiac injury through mTOR/ULK1-dependent autophagy. Oxid Med Cell Longev. 2018:5396806. DOI: 10.1155/2018/5396806. PMID: 29849901. PMCID: PMC5932983. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85055073043&origin=inward.
Article14. Lusha E, Jiang H. 2020; Simvastatin protects high glucose-induced H9c2 cells from injury by inducing autophagy. Pharm Biol. 58:1077–1084. DOI: 10.1080/13880209.2020.1839512. PMID: 33164619. PMCID: PMC7655079. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85095846970&origin=inward.15. Dewanjee S, Vallamkondu J, Kalra RS, John A, Reddy PH, Kandimalla R. 2021; Autophagy in the diabetic heart: a potential pharmacotherapeutic target in diabetic cardiomyopathy. Ageing Res Rev. 68:101338. DOI: 10.1016/j.arr.2021.101338. PMID: 33838320. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85104070130&origin=inward.
Article16. Li Y, Liu M, Song X, Zheng X, Yi J, Liu D, Wang S, Chu C, Yang J. 2020; Exogenous hydrogen sulfide ameliorates diabetic myocardial fibrosis by inhibiting cell aging through SIRT6/AMPK autophagy. Front Pharmacol. 11:1150. DOI: 10.3389/fphar.2020.01150. PMID: 32903815. PMCID: PMC7438924. PMID: 2a8dcd63b36943f59e9cef367eeedd7b. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85089492533&origin=inward.17. Yao Q, Ke ZQ, Guo S, Yang XS, Zhang FX, Liu XF, Chen X, Chen HG, Ke HY, Liu C. 2018; Curcumin protects against diabetic cardiomyopathy by promoting autophagy and alleviating apoptosis. J Mol Cell Cardiol. 124:26–34. DOI: 10.1016/j.yjmcc.2018.10.004. PMID: 30292723. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85054458374&origin=inward.
Article18. Tewari D, Patni P, Bishayee A, Sah AN, Bishayee A. 2022; Natural products targeting the PI3K-Akt-mTOR signaling pathway in cancer: a novel therapeutic strategy. Semin Cancer Biol. 80:1–17. DOI: 10.1016/j.semcancer.2019.12.008. PMID: 31866476. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85077381787&origin=inward.
Article19. Chen E, Chen C, Niu Z, Gan L, Wang Q, Li M, Cai X, Gao R, Katakam S, Chen H, Zhang S, Zhou R, Cheng X, Qiu Y, Yu H, Zhu T, Liu J. 2020; Poly(I:C) preconditioning protects the heart against myocardial ischemia/reperfusion injury through TLR3/PI3K/Akt-dependent pathway. Signal Transduct Target Ther. 5:216. DOI: 10.1038/s41392-020-00257-w. PMID: 33154351. PMCID: PMC7644758. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85095136454&origin=inward.
Article20. Ke X, Hao Y, Li B, Zou J, Li X, Wei C, Liu F, Zhang Z. 2018; Vaspin prevents tumor necrosis factor-α-induced apoptosis in cardiomyocytes by promoting autophagy. J Cardiovasc Pharmacol. 77:257–267. DOI: 10.1097/FJC.0000000000000562. PMID: 29734265. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85064269958&origin=inward.
Article21. Jia M, Qiu H, Lin L, Zhang S, Li D, Jin D. 2022; Inhibition of PI3K/AKT/mTOR signalling pathway activates autophagy and suppresses peritoneal fibrosis in the process of peritoneal dialysis. Front Physiol. 13:778479. DOI: 10.3389/fphys.2022.778479. PMID: 35309056. PMCID: PMC8931542. PMID: 619c8a1d8fad45e59f15312b7cbd03c7. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85127220061&origin=inward.
Article22. Lei L, Zhao J, Liu XQ, Chen J, Qi XM, Xia LL, Wu YG. 2021; Wogonin alleviates kidney tubular epithelial injury in diabetic nephropathy by inhibiting PI3K/Akt/NF-κB signaling pathways. Drug Des Devel Ther. 15:3131–3150. DOI: 10.2147/DDDT.S310882. PMID: 34295152. PMCID: PMC8291679. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85111455045&origin=inward.
Article23. Wang K, Li Z, Sun Y, Liu X, Ma W, Ding Y, Hong J, Qian L, Xu D. 2021; Dapagliflozin improves cardiac function, remodeling, myocardial apoptosis, and inflammatory cytokines in mice with myocardial infarction. J Cardiovasc Transl Res. doi:10.1007/s12265-021-10192-y. DOI: 10.1007/s12265-021-10192-y. PMID: 34855147. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85120464897&origin=inward.24. Yang X, Li X, Lin Q, Xu Q. 2019; Up-regulation of microRNA-203 inhibits myocardial fibrosis and oxidative stress in mice with diabetic cardiomyopathy through the inhibition of PI3K/Akt signaling pathway via PIK3CA. Gene. 715:143995. DOI: 10.1016/j.gene.2019.143995. PMID: 31336140. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85069924541&origin=inward.
Article25. Huang Y, Zhang H, Lv B, Tang C, Du J, Jin H. 2022; Sulfur dioxide: endogenous generation, biological effects, detection, and therapeutic potential. Antioxid Redox Signal. 36:256–274. DOI: 10.1089/ars.2021.0213. PMID: 34538110. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85123921067&origin=inward.
Article26. Liu M, Liu S, Tan W, Tang F, Long J, Li Z, Liang B, Chu C, Yang J. 2017; Gaseous signalling molecule SO2 via Hippo-MST pathway to improve myocardial fibrosis of diabetic rats. Mol Med Rep. 16:8953–8963. DOI: 10.3892/mmr.2017.7714. PMID: 28990064. PMCID: PMC5779980. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85032732375&origin=inward.
Article27. Zhang LL, Du JB, Tang CS, Jin HF, Huang YQ. 2018; Inhibitory effects of sulfur dioxide on rat myocardial fibroblast proliferation and migration. Chin Med J (Engl). 131:1715–1723. DOI: 10.4103/0366-6999.235875. PMID: 29998892. PMCID: PMC6048932. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85049839771&origin=inward.28. Wang JW, Ye XY, Wei N, Wu SS, Zhang ZH, Luo GH, Li X, Li J, Cao H. 2022; Reactive oxygen species contributes to type 2 diabetic neuropathic pain via the thioredoxin-interacting protein-NOD-like receptor protein 3- N -methyl-D-aspartic acid receptor 2B pathway. Anesth Analg. 135:865–876. DOI: 10.1213/ANE.0000000000006117. PMID: 35819160. PMCID: PMC9444295. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85138446377&origin=inward.
Article29. Yang L, Zhang H, Chen P. 2018; Sulfur dioxide attenuates sepsis-induced cardiac dysfunction via inhibition of NLRP3 inflammasome activation in rats. Nitric Oxide. 81:11–20. DOI: 10.1016/j.niox.2018.09.005. PMID: 30273666. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85054164749&origin=inward.
Article30. Li Z, Huang Y, Du J, Liu AD, Tang C, Qi Y, Jin H. 2016; Endogenous sulfur dioxide inhibits vascular calcification in association with the TGF-β/Smad signaling pathway. Int J Mol Sci. 17:266. DOI: 10.3390/ijms17030266. PMID: 26907267. PMCID: PMC4813130. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84975763787&origin=inward.
Article31. Zhang D, Wang X, Tian X, Zhang L, Yang G, Tao Y, Liang C, Li K, Yu X, Tang X, Tang C, Zhou J, Kong W, Du J, Huang Y, Jin H. 2018; The increased endogenous sulfur dioxide acts as a compensatory mechanism for the downregulated endogenous hydrogen sulfide pathway in the endothelial cell inflammation. Front Immunol. 9:882. DOI: 10.3389/fimmu.2018.00882. PMID: 29760703. PMCID: PMC5936987. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85046683013&origin=inward.
Article32. Han D, Rozanski A, Gransar H, Sharir T, Einstein AJ, Fish MB, Ruddy TD, Kaufmann PA, Sinusas AJ, Miller EJ, Bateman TM, Dorbala S, Di Carli M, Liang JX, Hu LH, Germano G, Dey D, Berman DS, Slomka PJ. 2020; Myocardial ischemic burden and differences in prognosis among patients with and without diabetes: results from the multicenter international REFINE SPECT registry. Diabetes Care. 43:453–459. DOI: 10.2337/dc19-1360. PMID: 31776140. PMCID: PMC6971784. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85078392590&origin=inward.
Article33. Shin SH, Claggett B, Pfeffer MA, Skali H, Liu J, Aguilar D, Diaz R, Dickstein K, Gerstein HC, Køber LV, Lawson FC, Lewis EF, Maggioni AP, McMurray JJV, Probstfield JL, Riddle MC, Tardif JC, Solomon SD. 2020; Hyperglycaemia, ejection fraction and the risk of heart failure or cardiovascular death in patients with type 2 diabetes and a recent acute coronary syndrome. Eur J Heart Fail. 22:1133–1143. DOI: 10.1002/ejhf.1790. PMID: 32212368. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85082750220&origin=inward.
Article34. Wamil M, Coleman RL, Adler AI, McMurray JJV, Holman RR. 2021; Increased risk of incident heart failure and death is associated with insulin resistance in people with newly diagnosed type 2 diabetes: UKPDS 89. Diabetes Care. 44:1877–1884. DOI: 10.2337/dc21-0429. PMID: 34162666. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85114206929&origin=inward.
Article35. Sacre JW, Magliano DJ, Shaw JE. 2020; Incidence of hospitalization for heart failure relative to major atherosclerotic events in type 2 diabetes: a meta-analysis of cardiovascular outcomes trials. Diabetes Care. 43:2614–2623. DOI: 10.2337/dc20-0654. PMID: 32958618. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85091472601&origin=inward.
Article36. Paulus WJ, Dal Canto E. 2018; Distinct myocardial targets for diabetes therapy in heart failure with preserved or reduced ejection fraction. JACC Heart Fail. 6:1–7. DOI: 10.1016/j.jchf.2017.07.012. PMID: 29284577. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85039972259&origin=inward.
Article37. Faria A, Persaud SJ. 2017; Cardiac oxidative stress in diabetes: mechanisms and therapeutic potential. Pharmacol Ther. 172:50–62. DOI: 10.1016/j.pharmthera.2016.11.013. PMID: 27916650. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85007448569&origin=inward.
Article38. Guido MC, Marques AF, Tavares ER, Tavares de Melo MD, Salemi VMC, Maranhão RC. 2017; The effects of diabetes induction on the rat heart: differences in oxidative stress, inflammatory cells, and fibrosis between subendocardial and interstitial myocardial areas. Oxid Med Cell Longev. 2017:5343972. DOI: 10.1155/2017/5343972. PMID: 28781721. PMCID: PMC5525092. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85026534841&origin=inward.
Article39. Li L, Zhao Q, Kong W. 2018; Extracellular matrix remodeling and cardiac fibrosis. Matrix Biol. 68-69:490–506. DOI: 10.1016/j.matbio.2018.01.013. PMID: 29371055. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85042052462&origin=inward.
Article40. Tuleta I, Frangogiannis NG. 2021; Fibrosis of the diabetic heart: clinical significance, molecular mechanisms, and therapeutic opportunities. Adv Drug Deliv Rev. 176:113904. DOI: 10.1016/j.addr.2021.113904. PMID: 34331987. PMCID: PMC8444077. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85111836439&origin=inward.
Article41. Wang CY, Li XD, Hao ZH, Xu D. 2016; Insulin-like growth factor-1 improves diabetic cardiomyopathy through antioxidative and anti-inflammatory processes along with modulation of Akt/GSK-3β signaling in rats. Korean J Physiol Pharmacol. 20:613–619. DOI: 10.4196/kjpp.2016.20.6.613. PMID: 27847438. PMCID: PMC5106395. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84995595683&origin=inward.42. Chiang MH, Liang CJ, Lin LC, Yang YF, Huang CC, Chen YH, Kao HL, Chen YC, Ke SR, Lee CW, Lin MS, Chen YL. 2020; miR-26a attenuates cardiac apoptosis and fibrosis by targeting ataxia-telangiectasia mutated in myocardial infarction. J Cell Physiol. 235:6085–6102. DOI: 10.1002/jcp.29537. PMID: 31990056. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85078656448&origin=inward.
Article43. Li J, Salvador AM, Li G, Valkov N, Ziegler O, Yeri A, Yang Xiao C, Meechoovet B, Alsop E, Rodosthenous RS, Kundu P, Huan T, Levy D, Tigges J, Pico AR, Ghiran I, Silverman MG, Meng X, Kitchen R, Xu J, et al. 2021; Mir-30d regulates cardiac remodeling by intracellular and paracrine signaling. Circ Res. 128:e1–e23. DOI: 10.1161/CIRCRESAHA.120.317244. PMID: 33092465. PMCID: PMC7790887. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85100280714&origin=inward.
Article44. Zou MH, Xie Z. 2013; Regulation of interplay between autophagy and apoptosis in the diabetic heart: new role of AMPK. Autophagy. 9:624–625. DOI: 10.4161/auto.23577. PMID: 23380689. PMCID: PMC3627682. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84876220612&origin=inward.
Article45. Das CK, Banerjee I, Mandal M. 2020; Pro-survival autophagy: an emerging candidate of tumor progression through maintaining hallmarks of cancer. Semin Cancer Biol. 66:59–74. DOI: 10.1016/j.semcancer.2019.08.020. PMID: 31430557. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85071228431&origin=inward.
Article46. Sciarretta S, Maejima Y, Zablocki D, Sadoshima J. 2018; The role of autophagy in the heart. Annu Rev Physiol. 80:1–26. DOI: 10.1146/annurev-physiol-021317-121427. PMID: 29068766. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85041929758&origin=inward.
Article47. Zhang M, Sui W, Xing Y, Cheng J, Cheng C, Xue F, Zhang J, Wang X, Zhang C, Hao P, Zhang Y. 2021; Angiotensin IV attenuates diabetic cardiomyopathy via suppressing FoxO1-induced excessive autophagy, apoptosis and fibrosis. Theranostics. 11:8624–8639. DOI: 10.7150/thno.48561. PMID: 34522203. PMCID: PMC8419053. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85112191000&origin=inward.48. Liu BY, Li L, Liu GL, Ding W, Chang WG, Xu T, Ji XY, Zheng XX, Zhang J, Wang JX. 2021; Baicalein attenuates cardiac hypertrophy in mice via suppressing oxidative stress and activating autophagy in cardiomyocytes. Acta Pharmacol Sin. 42:701–714. DOI: 10.1038/s41401-020-0496-1. PMID: 32796955. PMCID: PMC8115069. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85089387878&origin=inward.
Article49. Li X, Ke X, Li Z, Li B. 2019; Vaspin prevents myocardial injury in rats model of diabetic cardiomyopathy by enhancing autophagy and inhibiting inflammation. Biochem Biophys Res Commun. 514:1–8. DOI: 10.1016/j.bbrc.2019.04.110. PMID: 31014675. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85065791570&origin=inward.50. Wu MX, Wang SH, Xie Y, Chen ZT, Guo Q, Yuan WL, Guan C, Xu CZ, Huang YN, Wang JF, Zhang HF, Chen YX. 2021; Interleukin-33 alleviates diabetic cardiomyopathy through regulation of endoplasmic reticulum stress and autophagy via insulin-like growth factor-binding protein 3. J Cell Physiol. 236:4403–4419. DOI: 10.1002/jcp.30158. PMID: 33184863. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85096800458&origin=inward.
Article51. Magaye RR, Savira F, Hua Y, Xiong X, Huang L, Reid C, Flynn BL, Kaye D, Liew D, Wang BH. 2021; Attenuating PI3K/Akt- mTOR pathway reduces dihydrosphingosine 1 phosphate mediated collagen synthesis and hypertrophy in primary cardiac cells. Int J Biochem Cell Biol. 134:105952. DOI: 10.1016/j.biocel.2021.105952. PMID: 33609744. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85101654820&origin=inward.
Article52. Han Y, Cai X, Pan M, Gong J, Cai W, Lu D, Xu C. 2022; MicroRNA-21-5p acts via the PTEN/Akt/FOXO3a signaling pathway to prevent cardiomyocyte injury caused by high glucose/high fat conditions. Exp Ther Med. 23:230. DOI: 10.3892/etm.2022.11154. PMID: 35222707. PMCID: PMC8815051.
Article53. Wang XB, Huang XM, Ochs T, Li XY, Jin HF, Tang CS, Du JB. 2011; Effect of sulfur dioxide preconditioning on rat myocardial ischemia/reperfusion injury by inducing endoplasmic reticulum stress. Basic Res Cardiol. 106:865–878. DOI: 10.1007/s00395-011-0176-x. PMID: 21468766. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=80054689571&origin=inward.
Article54. Li Y, Feng Y, Ye X, Peng H, Du J, Yao X, Huang Y, Jin H, Du J. 2021; Endogenous SO2 controls cell apoptosis: the state-of-the-art. Front Cell Dev Biol. 9:729728. DOI: 10.3389/fcell.2021.729728. PMID: 34692686. PMCID: PMC8529009. PMID: 5412ed586591437d8610c242d9033e53. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85117532888&origin=inward.55. Chen Q, Zhang L, Chen S, Huang Y, Li K, Yu X, Wu H, Tian X, Zhang C, Tang C, Du J, Jin H. 2016; Downregulated endogenous sulfur dioxide/aspartate aminotransferase pathway is involved in angiotensin II-stimulated cardiomyocyte autophagy and myocardial hypertrophy in mice. Int J Cardiol. 225:392–401. DOI: 10.1016/j.ijcard.2016.09.111. PMID: 27770734. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84992202390&origin=inward.
Article56. Liang Y, Liu D, Ochs T, Tang C, Chen S, Zhang S, Geng B, Jin H, Du J. 2011; Endogenous sulfur dioxide protects against isoproterenol-induced myocardial injury and increases myocardial antioxidant capacity in rats. Lab Invest. 91:12–23. DOI: 10.1038/labinvest.2010.156. PMID: 20733562. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=78650776281&origin=inward.57. Wang XB, Cui H, Du JB. 2018; Sulfur dioxide ameliorates rat myocardial fibrosis by inhibiting endoplasmic reticulum stress. Histol Histopathol. 33:1089–1097. DOI: 10.14670/HH-18-007. PMID: 29851019. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85053180741&origin=inward.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Puerarin pretreatment attenuates cardiomyocyte apoptosis induced by coronary microembolization in rats by activating the PI3K/Akt/GSK-3β signaling pathway
- Dasatinib induces apoptosis and autophagy by suppressing the PI3K/Akt/mTOR pathway in bladder cancer cells
- Extracellular Ubiquitin Enhances Autophagy and Inhibits Mitochondrial Apoptosis Pathway to Protect Neurons Against Spinal Cord Ischemic Injury via CXCR4
- Hydrogen sulfide alleviates hypothyroidism-induced myocardial fibrosis in rats through stimulating autophagy and inhibiting TGF-β1/Smad2 pathway
- Hydrogen sulfide ameliorates abdominal aorta coarctationinduced myocardial fibrosis by inhibiting pyroptosis through regulating eukaryotic translation initiation factor 2αα phosphorylation and activating PI3K/AKT1 pathway