Korean J Pain.
2022 Oct;35(4):433-439. 10.3344/kjp.2022.35.4.433.
Spinal orexin A attenuates opioid-induced mechanical hypersensitivity in the rat
- Affiliations
-
- 1Department of Oral Physiology, School of Dentistry, Kyungpook National University, Daegu, Korea
- 2Advanced Dental Device Development Institute, School of Dentistry, Kyungpook National University, Daegu, Korea
- 3Department of Physiology, College of Veterinary Medicine, Kyungpook National University, Daegu, Korea
- 4Department of Anesthesiology and Pain Medicine, Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea
- KMID: 2533983
- DOI: http://doi.org/10.3344/kjp.2022.35.4.433
Abstract
- Background
Repeated administration of opioid analgesics for pain treatment can produce paradoxical hyperalgesia via peripheral and/or central mechanisms. Thus, this study investigated whether spinally (centrally) administered orexin A attenuates opioid-induced hyperalgesia (OIH).
Methods
[D-Ala2 , N-Me-Phe4 , Gly5 -ol]-enkephalin (DAMGO), a selective µ-opioid receptor agonist, was used to induce mechanical hypersensitivity and was administered intradermally (4 times, 1-hour intervals) on the rat hind paw dorsum. To determine whether post- or pretreatments with spinal orexin A, dynorphin A, and antidynorphin A were effective in OIH, the drugs were injected through an intrathecal catheter whose tip was positioned dorsally at the L3 segment of the spinal cord (5 µg for all). Mechanical hypersensitivity was assessed using von Frey monofilaments.
Results
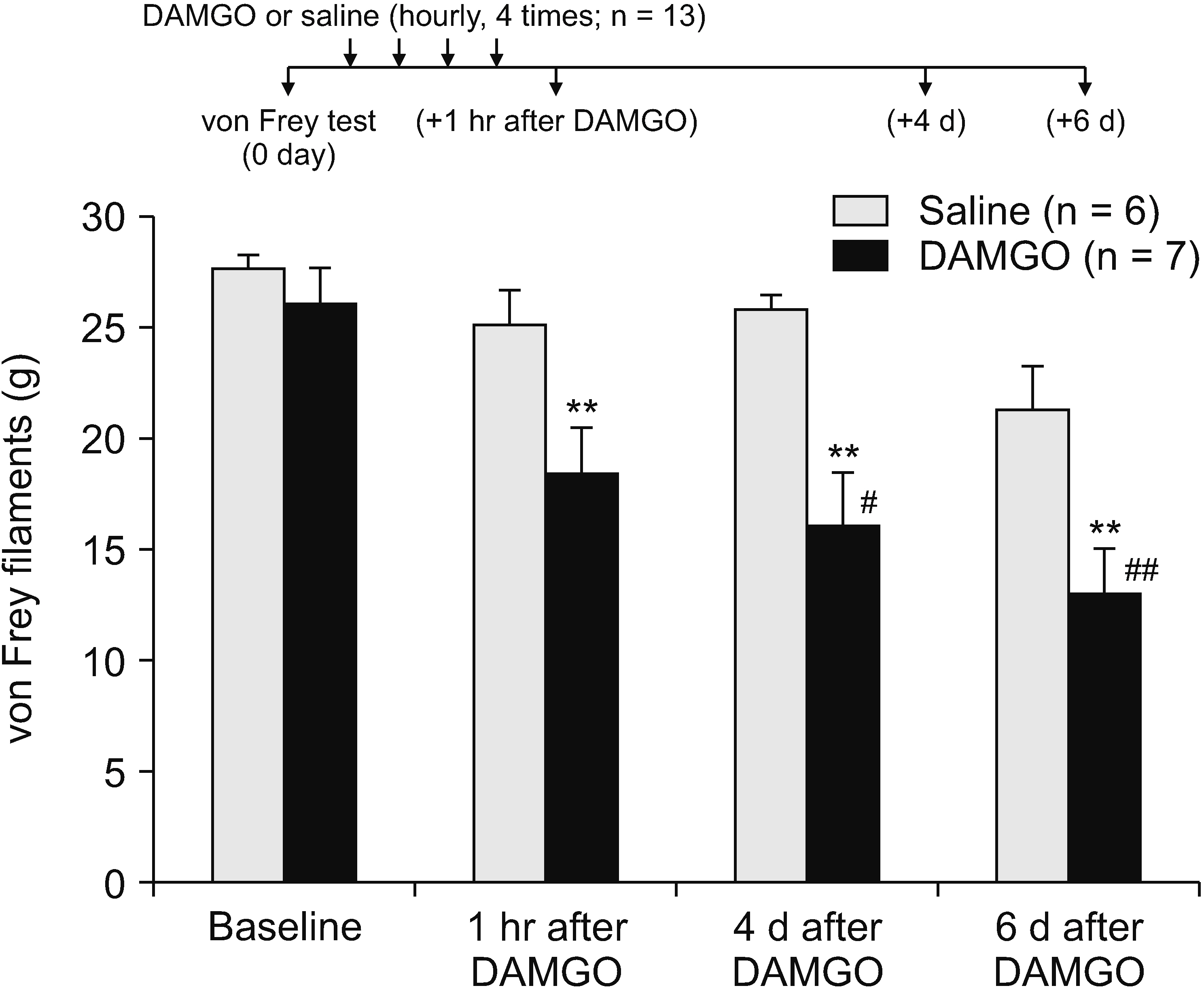
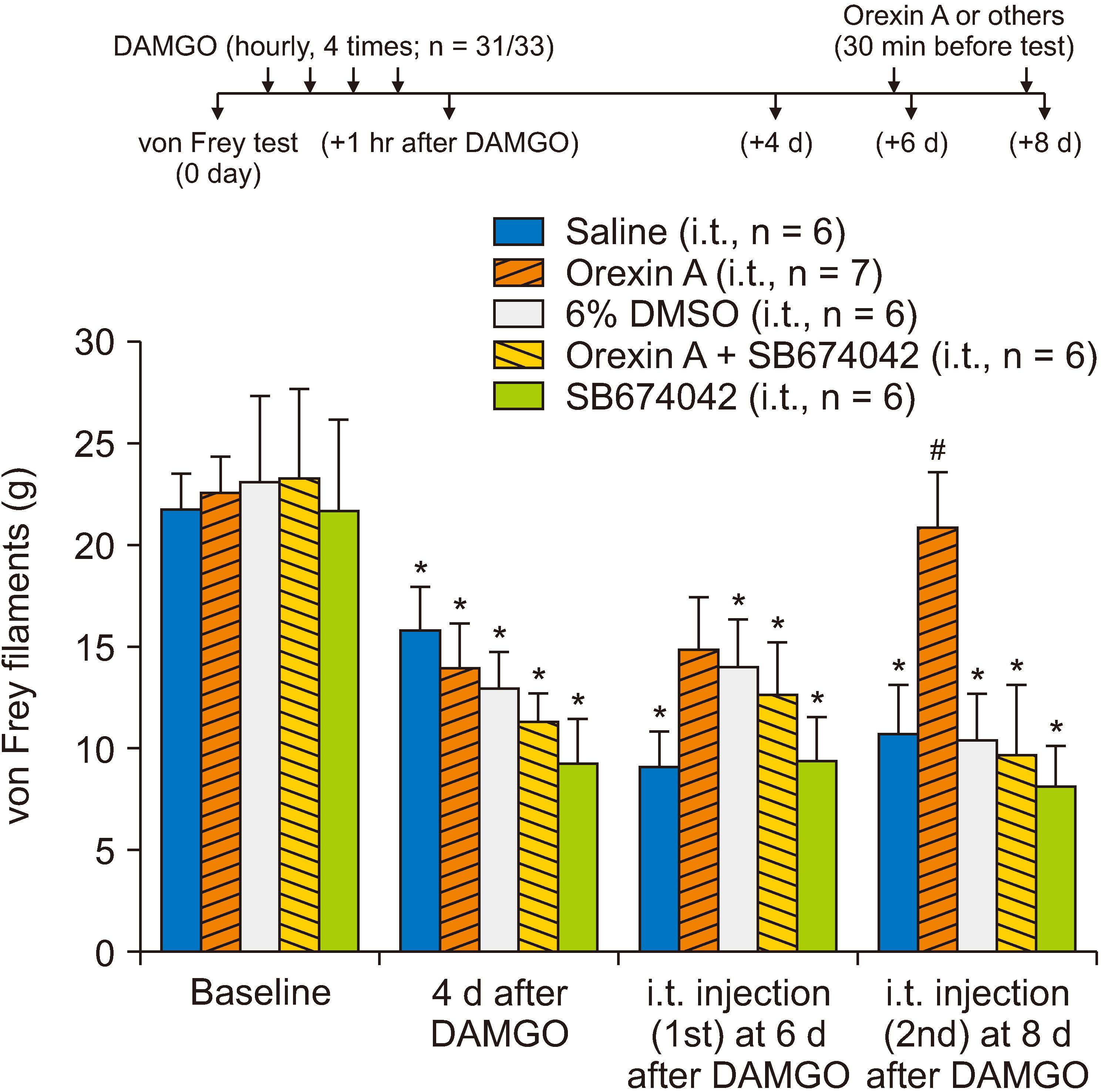
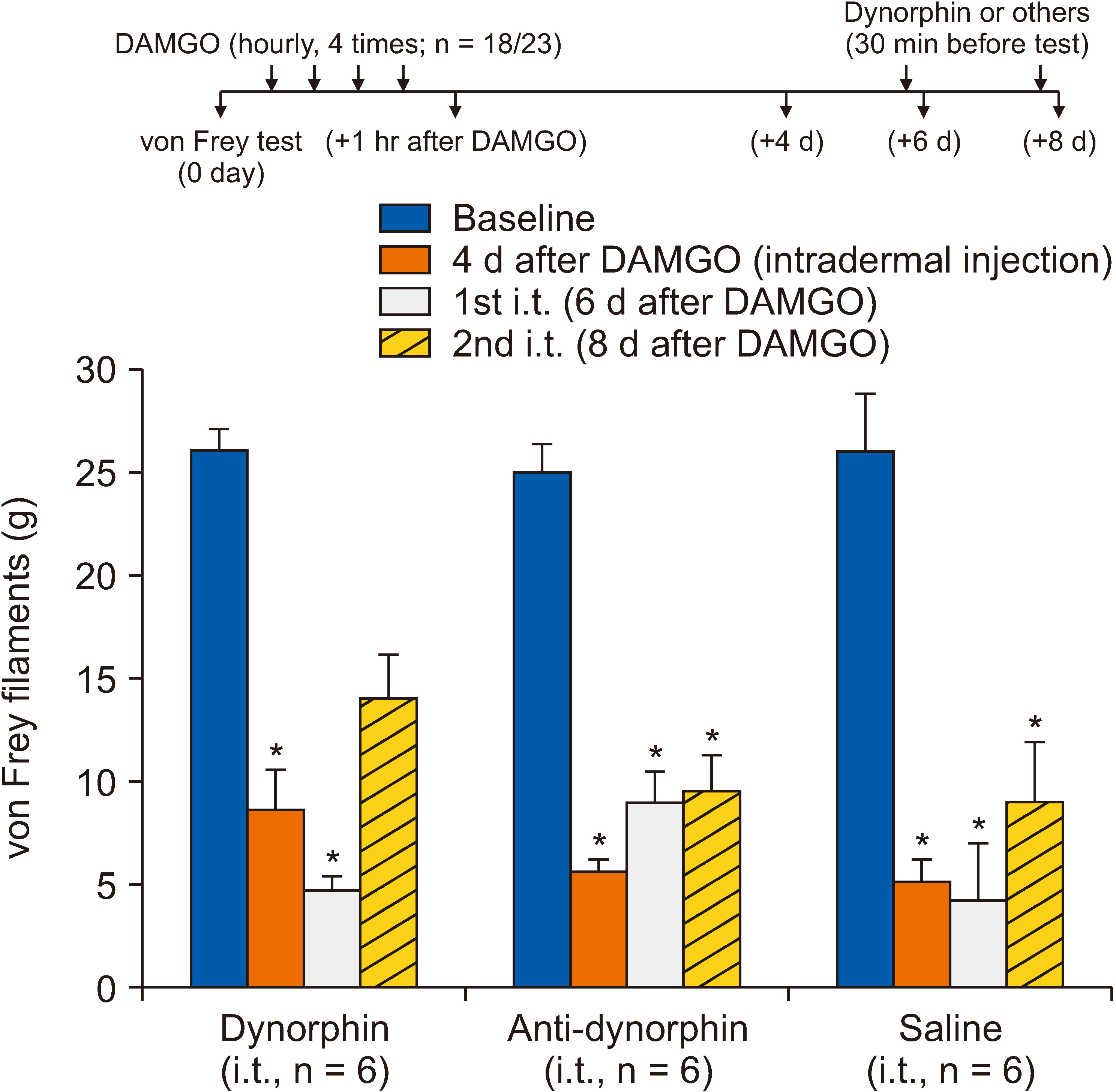
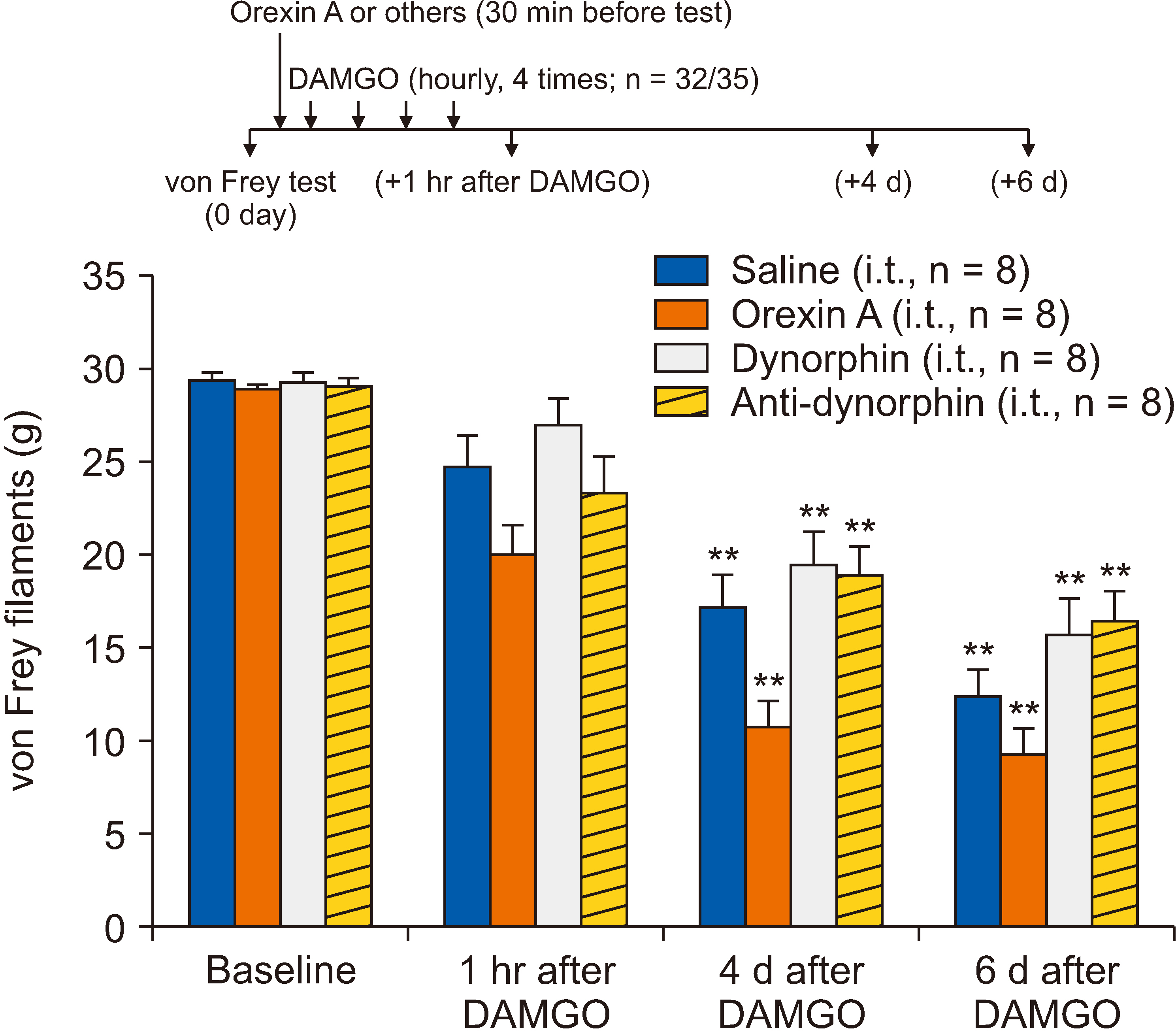
Repeated intradermal injections of DAMGO resulted in mechanical hypersensitivity in rats, lasting more than 8 days. Although the first intrathecal treatment of orexin A on the 6th day after DAMGO exposure did not show any significant effect on the mechanical threshold, the second (on the 8th day) significantly attenuated the DAMGO-induced mechanical hypersensitivity, which disappeared when the type 1 orexin receptor (OX1R) was blocked. However, intrathecal administration of dynorphin or an anti-dynorphin antibody (dynorphin antagonists) had no effect on DAMGO-induced hypersensitivity. Lastly, pretreatment with orexin A, dynorphin, or anti-dynorphin did not prevent DAMGO-induced mechanical hypersensitivity.
Conclusions
Spinal orexin A attenuates mechanical hyperalgesia induced by repetitive intradermal injections of DAMGO through OX1R. These data suggest that OIH can be potentially treated by activating the orexin A-OX1R pathway in the spinal dorsal horn.
Keyword
Figure
Cited by 1 articles
-
Nociplastic pain: controversy of the concept
Valdas Macionis
Korean J Pain. 2025;38(1):4-13. doi: 10.3344/kjp.24257.
Reference
-
1. Kim SH, Stoicea N, Soghomonyan S, Bergese SD. 2014; Intraoperative use of remifentanil and opioid induced hyperalgesia/acute opioid tolerance: systematic review. Front Pharmacol. 5:108. DOI: 10.3389/fphar.2014.00108. PMID: 24847273. PMCID: PMC4021143. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84904654274&origin=inward.
Article2. Lee M, Silverman SM, Hansen H, Patel VB, Manchikanti L. 2011; A comprehensive review of opioid-induced hyperalgesia. Pain Physician. 14:145–61. DOI: 10.36076/ppj.2011/14/145. PMID: 21412369.3. Mao J. 2002; Opioid-induced abnormal pain sensitivity: implications in clinical opioid therapy. Pain. 100:213–7. DOI: 10.1016/S0304-3959(02)00422-0. PMID: 12467992. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0036898884&origin=inward.
Article4. Mao J, Sung B, Ji RR, Lim G. 2002; Chronic morphine induces downregulation of spinal glutamate transporters: implications in morphine tolerance and abnormal pain sensitivity. J Neurosci. 22:8312–23. DOI: 10.1523/JNEUROSCI.22-18-08312.2002. PMID: 12223586. PMCID: PMC6758088. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0037107189&origin=inward.
Article5. Scholz J, Broom DC, Youn DH, Mills CD, Kohno T, Suter MR, et al. 2005; Blocking caspase activity prevents transsynaptic neuronal apoptosis and the loss of inhibition in lamina II of the dorsal horn after peripheral nerve injury. J Neurosci. 25:7317–23. DOI: 10.1523/JNEUROSCI.1526-05.2005. PMID: 16093381. PMCID: PMC6725303. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=23744437312&origin=inward.
Article6. Vanderah TW, Ossipov MH, Lai J, Malan TP Jr, Porreca F. 2001; Mechanisms of opioid-induced pain and antinociceptive tolerance: descending facilitation and spinal dynorphin. Pain. 92:5–9. DOI: 10.1016/S0304-3959(01)00311-6. PMID: 11323121. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0035063380&origin=inward.
Article7. Gardell LR, Wang R, Burgess SE, Ossipov MH, Vanderah TW, Malan TP Jr, et al. 2002; Sustained morphine exposure induces a spinal dynorphin-dependent enhancement of excitatory transmitter release from primary afferent fibers. J Neurosci. 22:6747–55. DOI: 10.1523/JNEUROSCI.22-15-06747.2002. PMID: 12151554. PMCID: PMC6758163. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0036703883&origin=inward.
Article8. Yamamoto Y, Ueta Y, Date Y, Nakazato M, Hara Y, Serino R, et al. 1999; Down regulation of the prepro-orexin gene expression in genetically obese mice. Brain Res Mol Brain Res. 65:14–22. DOI: 10.1016/S0169-328X(98)00320-9. PMID: 10036303. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0033582606&origin=inward.
Article9. Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, et al. 1999; The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 98:365–76. DOI: 10.1016/S0092-8674(00)81965-0. PMID: 10458611. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0033529520&origin=inward.
Article10. Date Y, Ueta Y, Yamashita H, Yamaguchi H, Matsukura S, Kangawa K, et al. 1999; Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc Natl Acad Sci U S A. 96:748–53. DOI: 10.1073/pnas.96.2.748. PMID: 9892705. PMCID: PMC15208. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0033582175&origin=inward.
Article11. Jeon Y, Park KB, Pervin R, Kim TW, Youn DH. 2015; Orexin-A modulates excitatory synaptic transmission and neuronal excitability in the spinal cord substantia gelatinosa. Neurosci Lett. 604:128–33. DOI: 10.1016/j.neulet.2015.08.001. PMID: 26254164. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84939240469&origin=inward.
Article12. Chen J, Sandkühler J. 2000; Induction of homosynaptic long-term depression at spinal synapses of sensory a delta-fibers requires activation of metabotropic glutamate receptors. Neuroscience. 98:141–8. DOI: 10.1016/S0306-4522(00)00080-4. PMID: 10858620. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0342979863&origin=inward.
Article13. Park KB, Weon H. 2017; Orexin receptors mediate long-term depression of excitatory synaptic transmission in the spinal cord dorsal horn. Neurosci Lett. 660:12–6. DOI: 10.1016/j.neulet.2017.08.068. PMID: 28866050. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85029174995&origin=inward.
Article14. Niknia S, Kaeidi A, Hajizadeh MR, Mirzaei MR, Khoshdel A, Hajializadeh Z, et al. 2019; Neuroprotective and antihyperalgesic effects of orexin-A in rats with painful diabetic neuropathy. Neuropeptides. 73:34–40. DOI: 10.1016/j.npep.2018.11.001. PMID: 30447858. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85056692005&origin=inward.
Article15. Yamamoto T, Saito O, Shono K, Aoe T, Chiba T. 2003; Anti-mechanical allodynic effect of intrathecal and intracerebroventricular injection of orexin-A in the rat neuropathic pain model. Neurosci Lett. 347:183–6. DOI: 10.1016/S0304-3940(03)00716-X. PMID: 12875916. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0043091918&origin=inward.
Article16. Risold PY, Griffond B, Kilduff TS, Sutcliffe JG, Fellmann D. 1999; Preprohypocretin (orexin) and prolactin-like immunoreactivity are coexpressed by neurons of the rat lateral hypothalamic area. Neurosci Lett. 259:153–6. DOI: 10.1016/S0304-3940(98)00906-9. PMID: 10025581. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0033555609&origin=inward.
Article17. Araldi D, Ferrari LF, Levine JD. 2015; Repeated mu-opioid exposure induces a novel form of the hyperalgesic priming model for transition to chronic pain. J Neurosci. 35:12502–17. DOI: 10.1523/JNEUROSCI.1673-15.2015. PMID: 26354917. PMCID: PMC4563038. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84941247747&origin=inward.
Article18. Yaksh TL, Rudy TA. 1976; Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 17:1031–6. DOI: 10.1016/0031-9384(76)90029-9. PMID: 14677603. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0017036162&origin=inward.
Article19. Deuis JR, Dvorakova LS, Vetter I. 2017; Methods used to evaluate pain behaviors in rodents. Front Mol Neurosci. 10:284. DOI: 10.3389/fnmol.2017.00284. PMID: 28932184. PMCID: PMC5592204. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85032342324&origin=inward.
Article20. Charan J, Kantharia ND. 2013; How to calculate sample size in animal studies? J Pharmacol Pharmacother. 4:303–6. DOI: 10.4103/0976-500X.119726. PMID: 24250214. PMCID: PMC3826013. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84886544464&origin=inward.
Article21. Wang Z, Gardell LR, Ossipov MH, Vanderah TW, Brennan MB, Hochgeschwender U, et al. 2001; Pronociceptive actions of dynorphin maintain chronic neuropathic pain. J Neurosci. 21:1779–86. DOI: 10.1523/JNEUROSCI.21-05-01779.2001. PMID: 11222667. PMCID: PMC6762963. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0035282798&origin=inward.
Article22. Vanderah TW, Laughlin T, Lashbrook JM, Nichols ML, Wilcox GL, Ossipov MH, et al. 1996; Single intrathecal injections of dynorphin A or des-Tyr-dynorphins produce long-lasting allodynia in rats: blockade by MK-801 but not naloxone. Pain. 68:275–81. DOI: 10.1016/S0304-3959(96)03225-3. PMID: 9121815. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=17344385566&origin=inward.
Article23. Mobarakeh JI, Takahashi K, Sakurada S, Nishino S, Watanabe H, Kato M, et al. 2005; Enhanced antinociception by intracerebroventricularly and intrathecally-administered orexin A and B (hypocretin-1 and -2) in mice. Peptides. 26:767–77. DOI: 10.1016/j.peptides.2005.01.001. PMID: 15808907. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=16244381170&origin=inward.
Article24. Cheng JK, Chou RC, Hwang LL, Chiou LC. 2003; Antiallodynic effects of intrathecal orexins in a rat model of postoperative pain. J Pharmacol Exp Ther. 307:1065–71. DOI: 10.1124/jpet.103.056663. PMID: 14551290. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0344304799&origin=inward.
Article25. Araldi D, Ferrari LF, Levine JD. 2017; Hyperalgesic priming (type II) induced by repeated opioid exposure: maintenance mechanisms. Pain. 158:1204–16. DOI: 10.1097/j.pain.0000000000000898. PMID: 28306605. PMCID: PMC5474187. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85021436988&origin=inward.
Article26. de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, et al. 1998; The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 95:322–7. DOI: 10.1073/pnas.95.1.322. PMID: 9419374. PMCID: PMC18213. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0008390266&origin=inward.27. Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. 1998; Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 92:573–85. DOI: 10.1016/S0092-8674(00)80949-6. PMID: 9527442. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=20244380014&origin=inward.
Article28. Cutler DJ, Morris R, Sheridhar V, Wattam TA, Holmes S, Patel S, et al. 1999; Differential distribution of orexin-A and orexin-B immunoreactivity in the rat brain and spinal cord. Peptides. 20:1455–70. DOI: 10.1016/S0196-9781(99)00157-6. PMID: 10698122. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0033370580&origin=inward.
Article29. van den Pol AN. 1999; Hypothalamic hypocretin (orexin): robust innervation of the spinal cord. J Neurosci. 19:3171–82. DOI: 10.1523/JNEUROSCI.19-08-03171.1999. PMID: 10191330. PMCID: PMC6782271. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0033561178&origin=inward.
Article30. Bingham S, Davey PT, Babbs AJ, Irving EA, Sammons MJ, Wyles M, et al. 2001; Orexin-A, an hypothalamic peptide with analgesic properties. Pain. 92:81–90. DOI: 10.1016/S0304-3959(00)00470-X. PMID: 11323129. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0035061724&origin=inward.
Article31. Hervieu GJ, Cluderay JE, Harrison DC, Roberts JC, Leslie RA. 2001; Gene expression and protein distribution of the orexin-1 receptor in the rat brain and spinal cord. Neuroscience. 103:777–97. DOI: 10.1016/S0306-4522(01)00033-1. PMID: 11274794. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0035925698&origin=inward.
Article32. Kimura M, Kawai Y, Ishikawa M, Shibutani K, Uchida R, Eguchi Y, et al. 2014; Effects of lidocaine and orexin on [Ca2+]i in dosal root ganglion neuron of rat segmental spinal nerve ligation model. Jpn Pharmacol Ther. 42:723–34. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0035925698&origin=inward.33. Zhou HY, Chen SR, Chen H, Pan HL. 2010; Opioid-induced long-term potentiation in the spinal cord is a presynaptic event. J Neurosci. 30:4460–6. DOI: 10.1523/JNEUROSCI.5857-09.2010. PMID: 20335482. PMCID: PMC2852319. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=77949839792&origin=inward.
Article34. Watanabe S, Kuwaki T, Yanagisawa M, Fukuda Y, Shimoyama M. 2005; Persistent pain and stress activate pain-inhibitory orexin pathways. Neuroreport. 16:5–8. DOI: 10.1097/00001756-200501190-00002. PMID: 15618879. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=13244273392&origin=inward.
Article35. Azhdari-Zarmehri H, Semnanian S, Fathollahi Y. 2015; Orexin-A modulates firing of rat rostral ventromedial medulla neurons: an in vitro study. Cell J. 17:163–70. DOI: 10.22074/cellj.2015.524. PMID: 25870847. PMCID: PMC4393666.36. Bannister K, Lee YS, Goncalves L, Porreca F, Lai J, Dickenson AH. 2014; Neuropathic plasticity in the opioid and non-opioid actions of dynorphin A fragments and their interactions with bradykinin B2 receptors on neuronal activity in the rat spinal cord. Neuropharmacology. 85:375–83. DOI: 10.1016/j.neuropharm.2014.06.005. PMID: 24937046. PMCID: PMC4873257. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84903195985&origin=inward.
Article37. Kajander KC, Sahara Y, Iadarola MJ, Bennett GJ. 1990; Dynorphin increases in the dorsal spinal cord in rats with a painful peripheral neuropathy. Peptides. 11:719–28. DOI: 10.1016/0196-9781(90)90187-A. PMID: 1978300. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0025142527&origin=inward.
Article38. Vanderah TW, Gardell LR, Burgess SE, Ibrahim M, Dogrul A, Zhong CM, et al. 2000; Dynorphin promotes abnormal pain and spinal opioid antinociceptive tolerance. J Neurosci. 20:7074–9. DOI: 10.1523/JNEUROSCI.20-18-07074.2000. PMID: 10995854. PMCID: PMC6772839. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0034666274&origin=inward.
Article39. Yamamoto T, Nozaki-Taguchi N, Chiba T. 2002; Analgesic effect of intrathecally administered orexin-A in the rat formalin test and in the rat hot plate test. Br J Pharmacol. 137:170–6. DOI: 10.1038/sj.bjp.0704851. PMID: 12208773. PMCID: PMC1573477. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0036740492&origin=inward.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Orexin and Alzheimer’s Disease: A New Perspective
- Dexmedetomidine Attenuates Neuropathic Pain by Inhibiting P2X7R Expression and ERK Phosphorylation in Rats
- The Mechanism of Antiallodynic Effect of Intrathecal Morphine in Neuropathic Pain Induced by Spinal Nerve Ligation
- The ability of orexin-A to modify pain-induced cyclooxygenase-2 and brain-derived neurotrophic factor expression is associated with its ability to inhibit capsaicin-induced pulpal nociception in rats
- Antiallodynic and anti-inflammatory effects of intrathecal R-PIA in a rat model of vincristine-induced peripheral neuropathy