J Pathol Transl Med.
2022 Jul;56(4):231-237. 10.4132/jptm.2022.05.08.
Primary pulmonary epithelioid inflammatory myofibroblastic sarcoma: a rare entity and a literature review
- Affiliations
-
- 1Department of Pathology, All India Institute of Medical Sciences, New Delhi, India
- 2Department of Surgical Oncology, Dr. BRA Institute Rotary Cancer Hospital, All India Institute of Medical Sciences, New Delhi, India
- 3Department of Medical Oncology, Dr. BRA Institute Rotary Cancer Hospital, All India Institute of Medical Sciences, New Delhi, India
- KMID: 2531616
- DOI: http://doi.org/10.4132/jptm.2022.05.08
Abstract
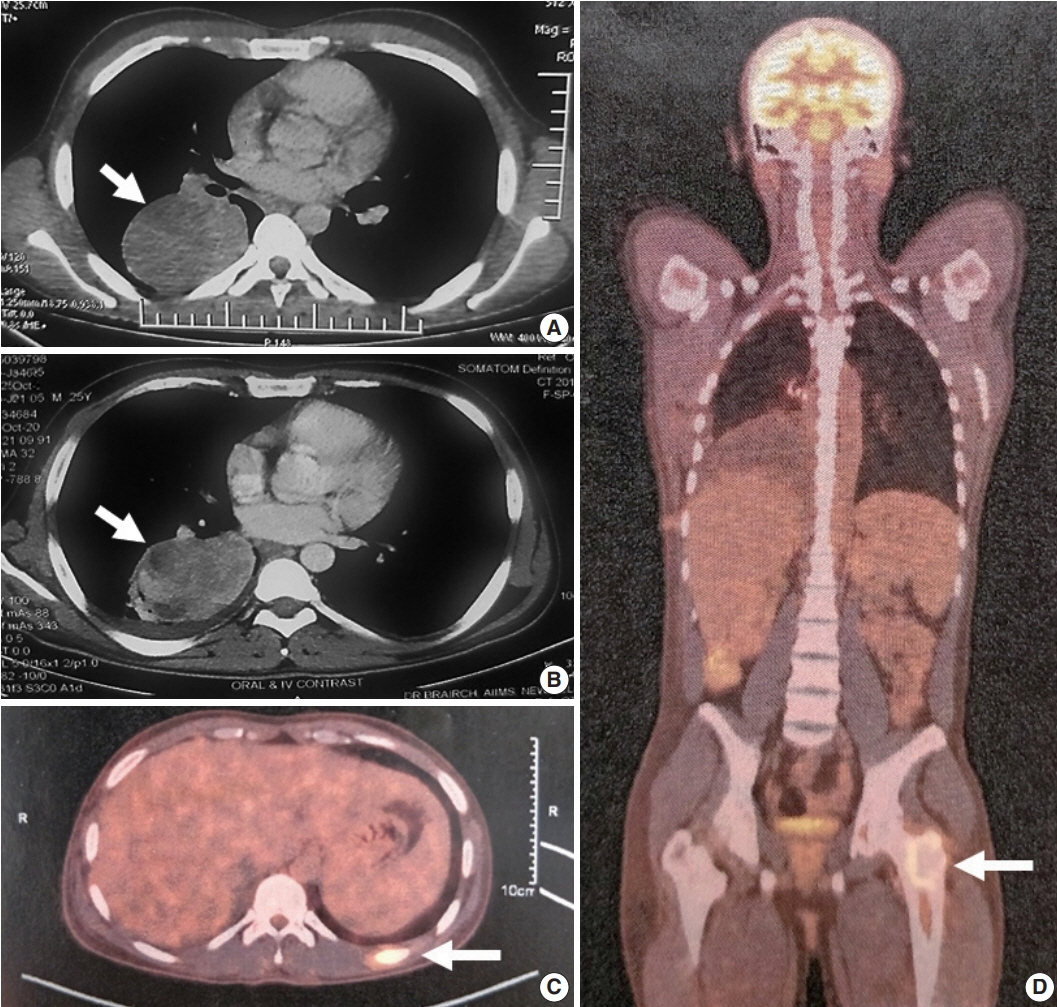
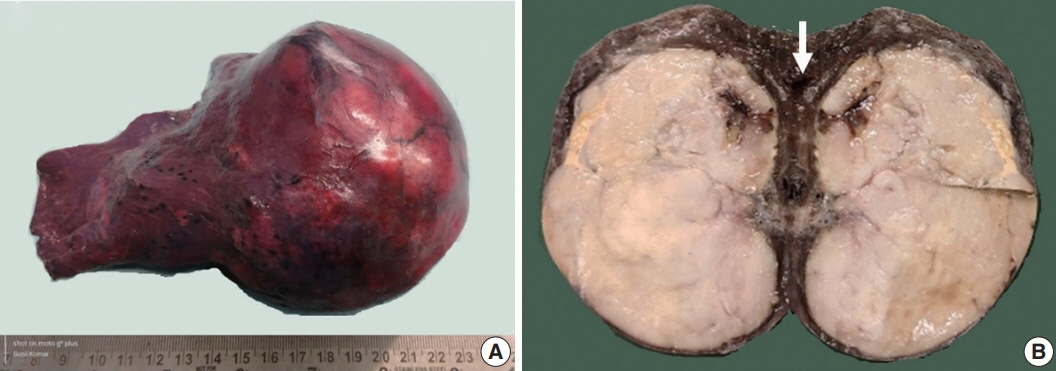
- Epithelioid inflammatory myofibroblastic sarcoma (EIMS) is an aggressive subtype of inflammatory myofibroblastic tumor (IMT) harboring anaplastic lymphoma kinase (ALK) gene fusions and is associated with high risk of local recurrence and poor prognosis. Herein, we present a young, non-smoking male who presented with complaints of cough and dyspnoea and was found to harbor a large right lower lobe lung mass. Biopsy showed a high-grade epithelioid to rhabdoid tumor with ALK and desmin protein expression. The patient initially received 5 cycles of crizotinib and remained stable for 1 year; however, he then developed multiple bony metastases, for which complete surgical resection was performed. Histopathology confirmed the diagnosis of EIMS, with ALK gene rearrangement demonstrated by fluorescence in situ hybridization. Postoperatively, the patient is asymptomatic with stable metastatic disease on crizotinib and has been started on palliative radiotherapy. EIMS is a very rare subtype of IMT that needs to be included in the differential diagnosis of ALKexpressing lung malignancies in young adults.
Keyword
Figure
Cited by 1 articles
-
Primary epithelioid inflammatory myofibroblastic sarcoma of the brain with
EML4::ALK fusion mimicking intra-axial glioma: a case report and brief literature review
Eric Eunshik Kim, Chul-Kee Park, Koung Mi Kang, Yoonjin Kwak, Sung-Hye Park, Jae-Kyung Won
J Pathol Transl Med. 2024;58(3):141-145. doi: 10.4132/jptm.2024.04.12.
Reference
-
References
1. Yamamoto H. Inflammatory myofibroblastic tumor. WHO Classification of Tumours Editorial Board. WHO classification of tumors: soft tissue and bone tumours. Lyon: IARC Press;2020. p. 109–11.2. Tavora F, Glass C, Hornick JL, Jain D, Sheppared MN, Yi ES. Inflammatory myofibroblastic tumor. WHO Classification of Tumours Editorial Board. WHO classification of tumors: thoracic tumours. Lyon: IARC Press;2021. p. 288–9.3. Marino-Enriquez A, Wang WL, Roy A, et al. Epithelioid inflammatory myofibroblastic sarcoma: an aggressive intra-abdominal variant of inflammatory myofibroblastic tumor with nuclear membrane or perinuclear ALK. Am J Surg Pathol. 2011; 35:135–44.4. Xu P, Shen P, Jin Y, Wang L, Wu W. Epithelioid inflammatory myofibroblastic sarcoma of stomach: diagnostic pitfalls and clinical characteristics. Int J Clin Exp Pathol. 2019; 12:1738–44.5. Lee JC, Li CF, Huang HY, et al. ALK oncoproteins in atypical inflammatory myofibroblastic tumours: novel RRBP1-ALK fusions in epithelioid inflammatory myofibroblastic sarcoma. J Pathol. 2017; 241:316–23.
Article6. Garg R, Kaul S, Arora D, Kashyap V. Posttransplant epithelioid inflammatory myofibroblastic sarcoma: a case report. Indian J Pathol Microbiol. 2019; 62:303–5.
Article7. Fu X, Jiang J, Tian XY, Li Z. Pulmonary epithelioid inflammatory myofibroblastic sarcoma with multiple bone metastases: case report and review of literature. Diagn Pathol. 2015; 10:106.
Article8. Sarmiento DE, Clevenger JA, Masters GA, Bauer TL, Nam BT. Epithelioid inflammatory myofibroblastic sarcoma: a case report. J Thorac Dis. 2015; 7:E513–6.9. Kozu Y, Isaka M, Ohde Y, Takeuchi K, Nakajima T. Epithelioid inflammatory myofibroblastic sarcoma arising in the pleural cavity. Gen Thorac Cardiovasc Surg. 2014; 62:191–4.
Article10. Yoshida A, Bubendorf L, Varella-Garcia M. ALK testing with FISH. In : Tsao MS, Hirsch FR, Yatabe Y, editors. IASLC atlas of ALK and ROS1 testing in lung cancer. 2nd ed. North Fort Myers: Editorial Rx Press;2016. p. 41–52.11. Parker BM, Parker JV, Lymperopoulos A, Konda V. A case report: pharmacology and resistance patterns of three generations of ALK inhibitors in metastatic inflammatory myofibroblastic sarcoma. J Oncol Pharm Pract. 2019; 25:1226–30.
Article12. Rush NI, Sinnott M. Malignant inflammatory myofibroblastic tumor of the lung with IgG4-positive plasma cells. Ann Clin Pathol. 2016; 4:1069.13. Du X, Shao Y, Qin HF, Tai YH, Gao HJ. ALK-rearrangement in non-small-cell lung cancer (NSCLC). Thorac Cancer. 2018; 9:423–30.14. Kim H, Jang SJ, Chung DH, et al. A comprehensive comparative analysis of the histomorphological features of ALK-rearranged lung adenocarcinoma based on driver oncogene mutations: frequent expression of epithelial-mesenchymal transition markers than other genotype. PLoS One. 2013; 8:e76999.
Article15. Inamura K, Takeuchi K, Togashi Y, et al. EML4-ALK lung cancers are characterized by rare other mutations, a TTF-1 cell lineage, an acinar histology, and young onset. Mod Pathol. 2009; 22:508–15.
Article16. Rodig SJ, Mino-Kenudson M, Dacic S, et al. Unique clinicopathologic features characterize ALK-rearranged lung adenocarcinoma in the western population. Clin Cancer Res. 2009; 15:5216–23.17. Yoshida A, Tsuta K, Watanabe S, et al. Frequent ALK rearrangement and TTF-1/p63 co-expression in lung adenocarcinoma with signet-ring cell component. Lung Cancer. 2011; 72:309–15.
Article18. Nishino M, Klepeis VE, Yeap BY, et al. Histologic and cytomorphologic features of ALK-rearranged lung adenocarcinomas. Mod Pathol. 2012; 25:1462–72.
Article19. Popat S, Gonzalez D, Min T, et al. ALK translocation is associated with ALK immunoreactivity and extensive signet-ring morphology in primary lung adenocarcinoma. Lung Cancer. 2012; 75:300–5.
Article20. Cook JR, Dehner LP, Collins MH, et al. Anaplastic lymphoma kinase (ALK) expression in the inflammatory myofibroblastic tumor: a comparative immunohistochemical study. Am J Surg Pathol. 2001; 25:1364–71.21. Campo E, Gascoyne RD. ALK-positive large B-cell lymphoma. In : Swerdlow SH, Campo E, Harris NL, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. Revised 4th ed. Lyon: IARC Press;2017. p. 319–20.22. Busam KJ, Vilain RE, Lum T, et al. Primary and metastatic cutaneous melanomas express ALK through alternative transcriptional initiation. Am J Surg Pathol. 2016; 40:786–95.
Article23. Couts KL, Bemis J, Turner JA, et al. ALK inhibitor response in melanomas expressing EML4-ALK fusions and alternate ALK isoforms. Mol Cancer Ther. 2018; 17:222–31.24. Cessna MH, Zhou H, Sanger WG, et al. Expression of ALK1 and p80 in inflammatory myofibroblastic tumor and its mesenchymal mimics: a study of 135 cases. Mod Pathol. 2002; 15:931–8.
Article25. Gasparini P, Casanova M, Villa R, et al. Anaplastic lymphoma kinase aberrations correlate with metastatic features in pediatric rhabdomyosarcoma. Oncotarget. 2016; 7:58903–14.
Article26. Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007; 448:561–6.
Article27. Kim H, Chung JH. Overview of clinicopathologic features of ALKrearranged lung adenocarcinoma and current diagnostic testing for ALK rearrangement. Transl Lung Cancer Res. 2015; 4:149–55.28. Fabre D, Fadel E, Singhal S, et al. Complete resection of pulmonary inflammatory pseudotumors has excellent long-term prognosis. J Thorac Cardiovasc Surg. 2009; 137:435–40.
Article29. Sakurai H, Hasegawa T, Watanabe S, Suzuki K, Asamura H, Tsuchiya R. Inflammatory myofibroblastic tumor of the lung. Eur J Cardiothorac Surg. 2004; 25:155–9.
Article30. Childress MA, Himmelberg SM, Chen H, Deng W, Davies MA, Lovly CM. ALK fusion partners impact response to ALK inhibition: differential effects on sensitivity, cellular phenotypes, and biochemical properties. Mol Cancer Res. 2018; 16:1724–36.
Article31. Fordham AM, Xie J, Gifford AJ, et al. CD30 and ALK combination therapy has high therapeutic potency in RANBP2-ALK-rearranged epithelioid inflammatory myofibroblastic sarcoma. Br J Cancer. 2020; 123:1101–13.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Primary epithelioid inflammatory myofibroblastic sarcoma of the brain with EML4::ALK fusion mimicking intra-axial glioma: a case report and brief literature review
- A case of epithelioid sarcoma arising in the vulva
- Inflammatory Myofibroblastic Tumor of Nasal Septum after Septoplasty: A Case Report
- A Case of Epithelioid Sarcoma in a Child
- A Case Report of Cutaneous Low-grade Myofibroblastic Sarcoma in the Neck