Korean J Health Promot.
2022 Jun;22(2):62-67. 10.15384/kjhp.2022.22.2.62.
Evaluation of COVID-19 Biokit IgG/IgM Clinical Effectiveness in COVID-19 Vaccinated Individuals
- Affiliations
-
- 1Department of Family Medicine, Soonchunhyang University Hospital Seoul, Seoul, Korea
- KMID: 2531101
- DOI: http://doi.org/10.15384/kjhp.2022.22.2.62
Abstract
- Background
This study compared the neutralizing antibody kit using the Enzyme-Linked Immunosorbent Assay (ELISA) method with the rapid antibody diagnostic kit using the Lateral Flow Immunoassay (LFIA) method to evaluate the clinical effectiveness of the COVID-19 Biokit IgG/IgM regarding evaluation of antibody formation after COVID-19 vaccination.
Methods
The neutralizing antibody test was performed with antibody detection kit of diagnostic medical devices for the qualitative method using the standard ELISA method. The rapid antibody diagnostic kit was measured with the COVID-19 Biokit IgG/IgM using the LFIA method. Based on the results of the neutralizing antibody measurement test of the standard test method, the test results of the rapid antibody diagnostic kit are compared and analyzed to confirm its the sensitivity and specificity.
Results
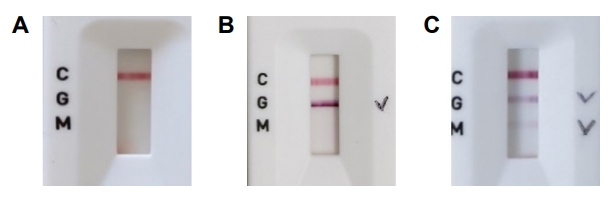
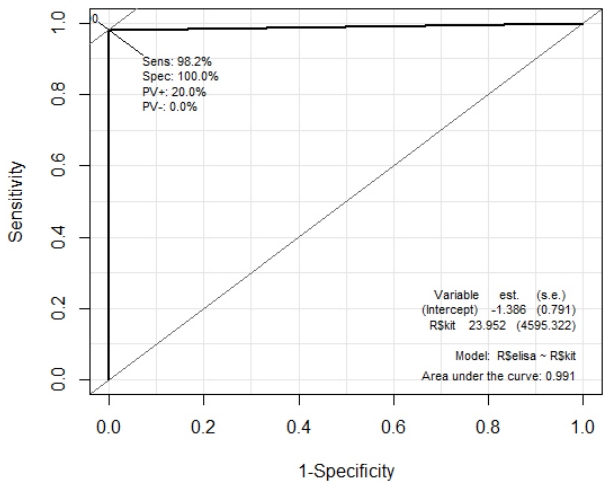
When the consistency was determined as positive and negative for the two test results, 118 cases were matched and two cases were inconsistent, showing a 98.3% consistency rate. That is, sensitivity 98%, specificity 100% and correctly classified proportion 98%.
Conclusions
Although the positive results of antibody formation of this kit would mean that individual has immunity to COVID-19, the result cannot be used to confirm or evaluate for re-infection. But the strong agreement between rapid antibody diagnostic kit results and ELISA results suggests that the kit used in this study is available as a screening test for antibody and neutralizing antibody responses, which could help evaluate the need for additional vaccinations, collect data quickly and cheaply and monitor individual immune responses.
Keyword
Figure
Reference
-
1. Centers for Disease Control and Prevention (CDC). Coronavirus Disease 2019 (COVID-19) [Internet]. Atlanta: CDC; 2021 [cited Apr 28, 2022]. Available from: https://www.cdc.gov/dotw/covid-19/index.html.2. World Health Organization (WHO). WHO Coronavirus (COVID-19) Dashboard [Internet]. Geneva: WHO; 2022 [cited Apr 1, 2022]. Available from: https://covid19.who.int/.3. Heyming TW, Nugent D, Tongol A, Knudsen-Robbins C, Hoang J, Schomberg J, et al. Rapid antibody testing for SARS-CoV-2 vaccine response in pediatric healthcare workers. Int J Infect Dis. 2021; 113:1–6.
Article4. Johns Hopkins Center For Health Security, COVID-19 Testing Toolkit. Serology tests for COVID-19 [Internet]. Baltimore: Johns Hopkins Center for Health Security, COVID-19 Testing Toolkit; 2022 [cited Apr 28, 2022]. Available from: https://www.centerforhealthsecurity.org/covid-19TestingToolkit/serology/Serology-based-tests-for-COVID-19.html.5. West R, Kobokovich A, Connell N, Gronvall GK. COVID-19 antibody tests: a valuable public health tool with limited relevance to individuals. Trends Microbiol. 2021; 29(3):214–23.
Article6. Seow J, Graham C, Merrick B, Acors S, Pickering S, Steel KJA, et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat Microbiol. 2020; 5(12):1598–607.
Article7. Post N, Eddy D, Huntley C, van Schalkwyk MCI, Shrotri M, Leeman D, et al. Antibody response to SARS-CoV-2 infection in humans: a systematic review. PLoS One. 2020; 15(12):e0244126.
Article8. Sullivan PS, Sailey C, Guest JL, Guarner J, Kelley C, Siegler AJ, et al. Detection of SARS-CoV-2 RNA and antibodies in diverse samples: protocol to validate the sufficiency of provider-observed, home-collected blood, saliva, and oropharyngeal samples. JMIR Public Health Surveill. 2020; 6(2):e19054.
Article9. Gronvall G, Connell N, Kobokovich A, West R, Warmbrod KL, Shearer MP, et al. Developing a national strategy for serology (antibody testing) in the United States. Baltimore: The Johns Hopkins Center for Health Security; 2020.10. Addetia A, Crawford KHD, Dingens A, Zhu H, Roychoudhury P, Huang ML, et al. Neutralizing antibodies correlate with protection from SARS-CoV-2 in humans during a fishery vessel outbreak with a high attack rate. J Clin Microbiol. 2020; 58(11):e02107-20.
Article11. Choi DO, Lee KM. Development of COVID-19 neutralizing antibody (NAb) detection kits using the S1 RBD protein of SARS-CoV-2. KJCLS. 2021; 53:257–65.
Article12. Lee S. The usefulness of the COVID-19 rapid diagnosis kit. Korean J Healthc Assoc Infect Control Prev. 2021; 26(2):134–6.
Article13. Kim N, Minn D, Park S, Roh EY, Yoon JH, Park H, et al. Positivity of SARS-CoV-2 antibodies among Korean healthy healthcare workers 1 and 2 weeks after second dose of Pfizer-BioNTech vaccination. J Korean Med Sci. 2021; 36(21):e158.
Article14. Sethuraman N, Jeremiah SS, Ryo A. Interpreting diagnostic tests for SARS-CoV-2. JAMA. 2020; 323(22):2249–51.
Article15. Centers for Disease Control and Prevention (CDC). Antibody Testing Guidelines [Internet]. Atlanta: CDC; 2022 [cited Apr 28, 2022]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antibody-tests-guidelines.html.16. Jarrom D, Elston L, Washington J, Prettyjohns M, Cann K, Myles S, et al. Effectiveness of tests to detect the presence of SARS-CoV-2 virus, and antibodies to SARS-CoV-2, to inform COVID-19 diagnosis: a rapid systematic review. BMJ Evid Based Med. 2022; 27(1):33–45.
Article17. EUROPEAN COMMISSION. Communication from the Commission, Guidelines on COVID-19 in vitro diagnostic tests and their performance. 15th ed. 4. Brussels: EUROPEAN COMMISSION;2020.18. American Association for Clinical Chemistry (AACC). AACC Recommendations for SARS-CoV-2 Serology Testing [Internet]. Washington, DC: AACC; 2020 [cited Apr 28, 2022]. Available from: https://www.aacc.org/science-and-research/covid-19-resources/statements-on-covid-19-testing/aacc-recommendations-for-sars-cov-2-serology-testing.19. Infectious Diseases Society of America (IDSA). IDSA COVID-19 Antibody Testing Primer [Internet]. New York: IDSA; 2020 [cited Apr 28, 2022]. Available from: https://www.idsociety.org/globalassets/idsa/public-health/covid-19/idsacovid-19-antibody-testing-primer.pdf.20. Wang Z, Muecksch F, Schaefer-Babajew D, Finkin S, Viant C, Gaebler C, et al. Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection. Nature. 2021; 595(7867):426–31.
Article21. Widge AT, Rouphael NG, Jackson LA, Anderson EJ, Roberts PC, Makhene M, et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N Engl J Med. 2021; 384(1):80–2.
Article22. Mahmoud SA, Ganesan S, Naik S, Bissar S, Zamel IA, Warren KN, et al. Serological assays for assessing postvaccination SARS-CoV-2 antibody response. Microbiol Spectr. 2021; 9(2):e0073321.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Difference in Anti-nucleocapsid Protein Antibody Responses Between Vaccinated and Unvaccinated Individuals After Asymptomatic, Mild, or Moderate COVID-19 Infection
- Evaluation of Clinical and Analytical Performance of a Nucleocapsid Protein Antigenbased ELISA for Detecting Antibodies against SARS-CoV-2
- Impact of solid organ transplantation on the effectiveness of COVID-19 vaccination in hospitalized patients with COVID-19: a propensity score-matched cohort study
- The Management of Thyroid Disease in COVID-19 Pandemic
- Delayed exacerbation of COVID-19 pneumonia in vaccinated kidney transplant recipients receiving immunosuppressants: a case series