Korean J Physiol Pharmacol.
2022 Jul;26(4):287-295. 10.4196/kjpp.2022.26.4.287.
Adipose-derived stem cells decolonize skin Staphylococcus aureus by enhancing phagocytic activity of peripheral blood mononuclear cells in the atopic rats
- Affiliations
-
- 1Neuroscience Research Institute and Department of Physiology, Korea University College of Medicine, Seoul 02841, Korea
- 2Glovi Plastic Surgery, Seoul 06031, Korea
- 3Department of Microbiology, Korea University College of Medicine, Seoul 02841, Korea
- 4Division of Biological Science and Technology, Science and Technology College, Yonsei University Mirae Campus, Wonju 26493, Korea
- 5Department of Biomedical Laboratory Science, College of Medical Science, Konyang University, Daejeon 35365, Korea
- KMID: 2530949
- DOI: http://doi.org/10.4196/kjpp.2022.26.4.287
Abstract
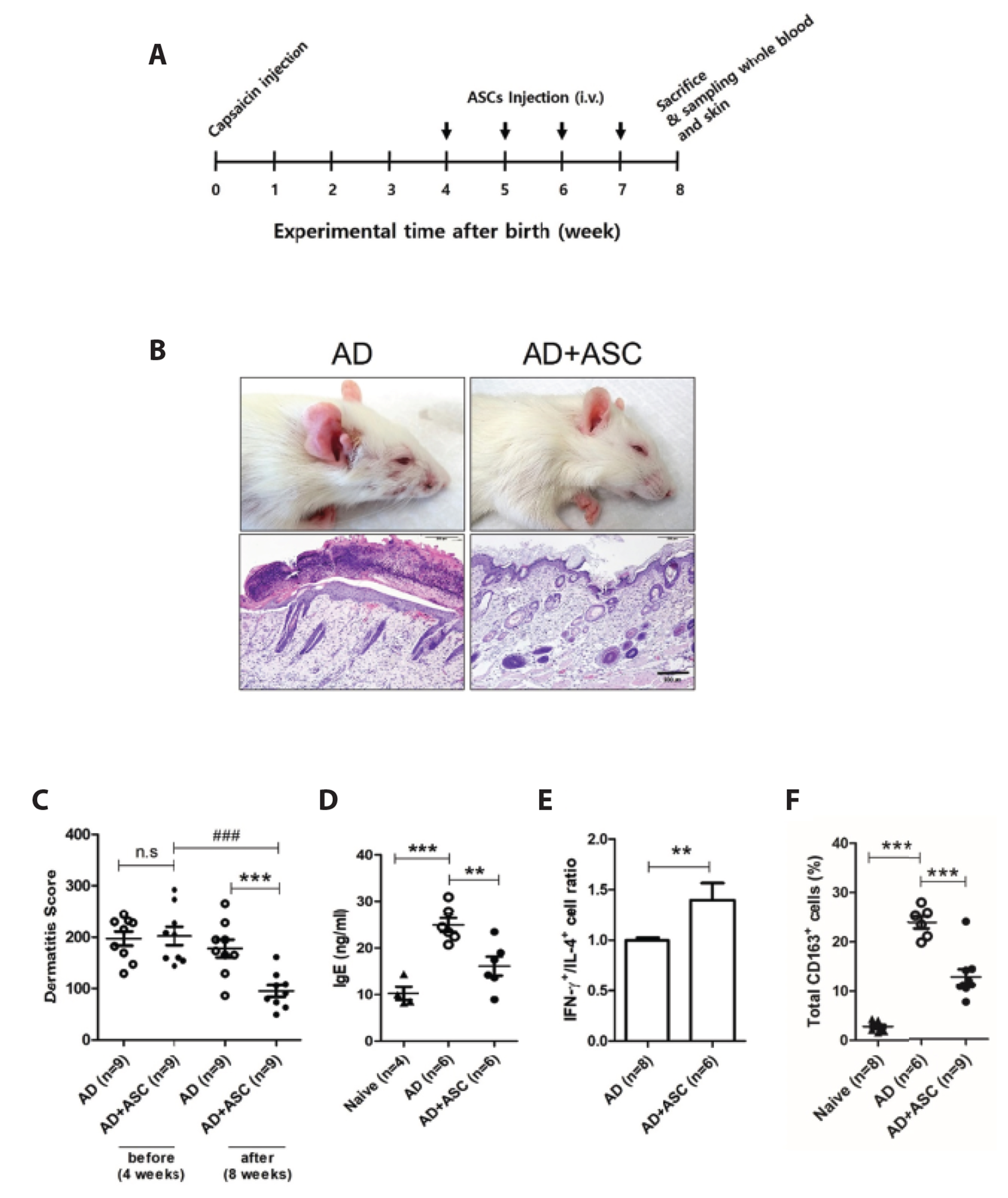
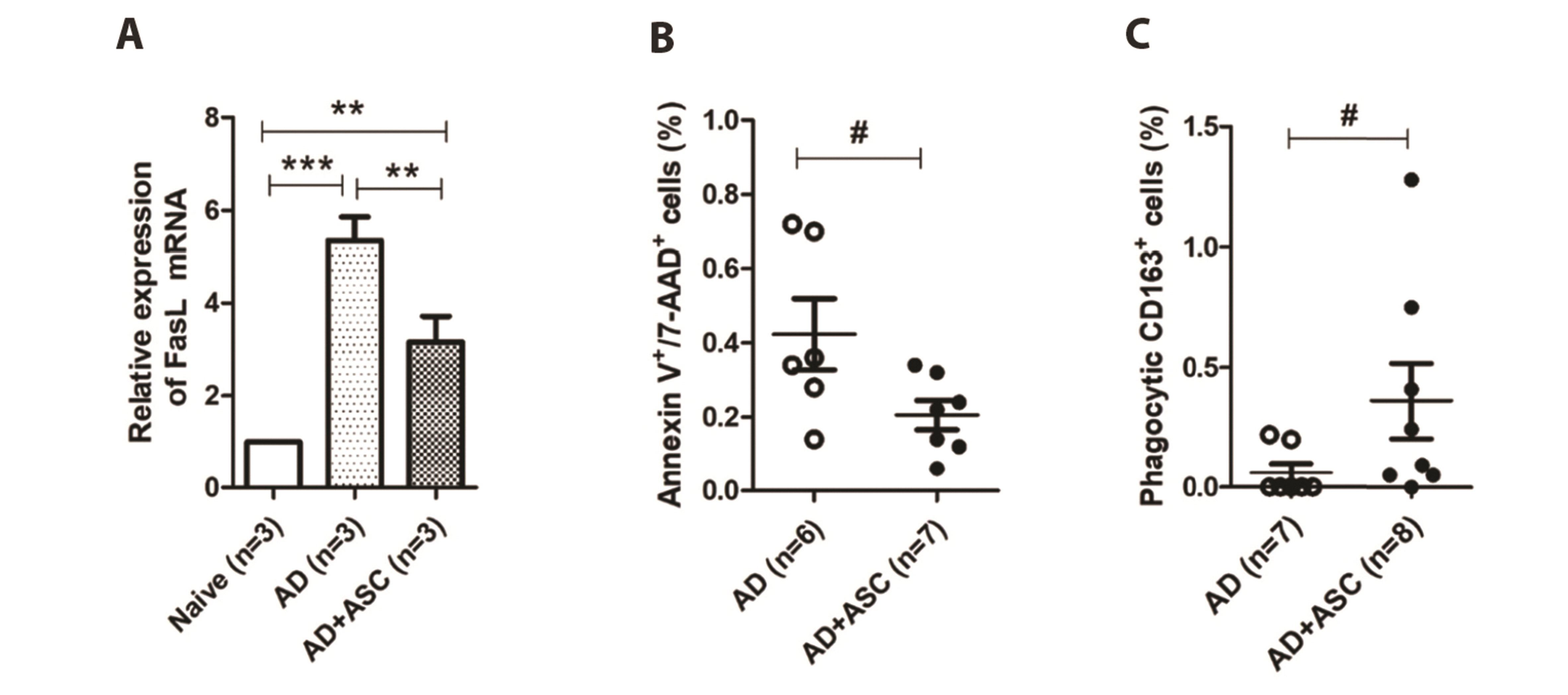
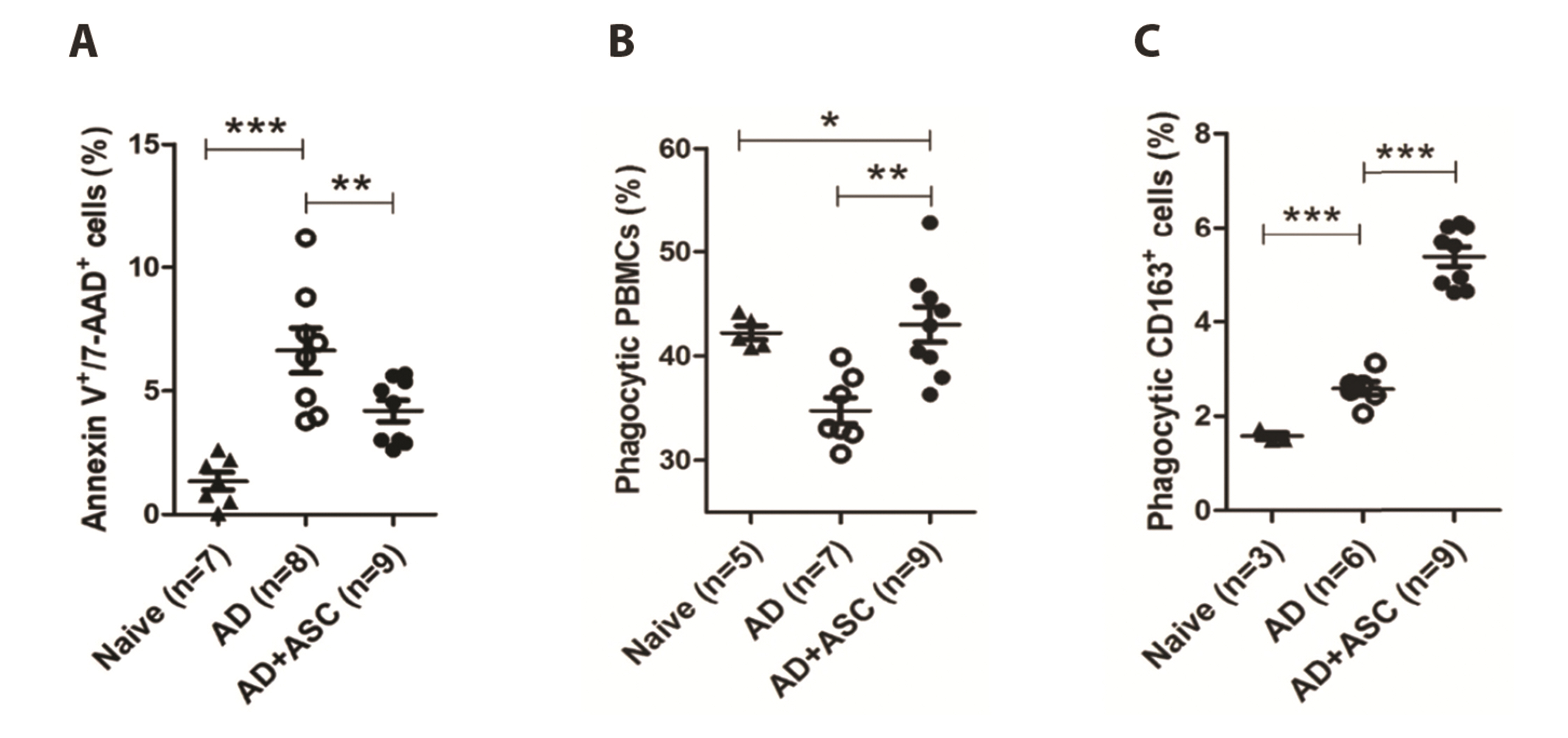
- Staphylococcus aureus (S. aureus ) is known to induce apoptosis of host immune cells and impair phagocytic clearance, thereby being pivotal in the pathogenesis of atopic dermatitis (AD). Adipose-derived stem cells (ASCs) exert therapeutic effects against inflammatory and immune diseases. In the present study, we investigated whether systemic administration of ASCs restores the phagocytic activity of peripheral blood mononuclear cells (PBMCs) and decolonizes cutaneous S. aureus under AD conditions. AD was induced by injecting capsaicin into neonatal rat pups. ASCs were extracted from the subcutaneous adipose tissues of naïve rats and administered to AD rats once a week for a month. Systemic administration of ASCs ameliorated AD-like symptoms, such as dermatitis scores, serum IgE, IFN-γ+/IL-4+ cell ratio, and skin colonization by S. aureus in AD rats. Increased FasL mRNA and annexin V+/7-AAD+ cells in the PBMCs obtained from AD rats were drastically reversed when co-cultured with ASCs. In contrast, both PBMCs and CD163+ cells bearing fluorescent zymosan particles significantly increased in AD rats treated with ASCs. Additionally, the administration of ASCs led to an increase in the mRNA levels of antimicrobial peptides, such as cathelicidin and β-defensin, in the skin of AD rats. Our results demonstrate that systemic administration of ASCs led to decolonization of S. aureus by attenuating apoptosis of immune cells in addition to restoring phagocytic activity. This contributes to the improvement of skin conditions in AD rats. Therefore, administration of ASCs may be helpful in the treatment of patients with intractable AD.
Keyword
Figure
Reference
-
1. Leung DY, Bieber T. 2003; Atopic dermatitis. Lancet. 361:151–160. DOI: 10.1016/S0140-6736(03)12193-9. PMID: 32738956. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0037431756&origin=inward.
Article2. Kasraie S, Werfel T. 2013; Role of macrophages in the pathogenesis of atopic dermatitis. Mediators Inflamm. 2013:942375. DOI: 10.1155/2013/942375. PMID: 23533313. PMCID: PMC3603294. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84875459401&origin=inward.
Article3. Zhang X, Hu X, Rao X. 2017; Apoptosis induced by Staphylococcus aureus toxins. Microbiol Res. 205:19–24. DOI: 10.1016/j.micres.2017.08.006. PMID: 28942840. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85027963050&origin=inward.
Article4. Torchinsky MB, Garaude J, Blander JM. 2010; Infection and apoptosis as a combined inflammatory trigger. Curr Opin Immunol. 22:55–62. DOI: 10.1016/j.coi.2010.01.003. PMID: 20137905. PMCID: PMC5800876. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=77249170723&origin=inward.
Article5. Deng W, Chen W, Zhang Z, Huang S, Kong W, Sun Y, Tang X, Yao G, Feng X, Chen W, Sun L. 2015; Mesenchymal stem cells promote CD206 expression and phagocytic activity of macrophages through IL-6 in systemic lupus erythematosus. Clin Immunol. 161:209–216. DOI: 10.1016/j.clim.2015.07.011. PMID: 26209923. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84942582111&origin=inward.
Article6. Poon IK, Lucas CD, Rossi AG, Ravichandran KS. 2014; Apoptotic cell clearance: basic biology and therapeutic potential. Nat Rev Immunol. 14:166–180. DOI: 10.1038/nri3607. PMID: 24481336. PMCID: PMC4040260. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84896690342&origin=inward.
Article7. Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. 2002; Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 13:4279–4295. DOI: 10.1091/mbc.e02-02-0105. PMID: 12475952. PMCID: PMC138633. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=18744373595&origin=inward.
Article8. Valina C, Pinkernell K, Song YH, Bai X, Sadat S, Campeau RJ, Le Jemtel TH, Alt E. 2007; Intracoronary administration of autologous adipose tissue-derived stem cells improves left ventricular function, perfusion, and remodelling after acute myocardial infarction. Eur Heart J. 28:2667–2677. DOI: 10.1093/eurheartj/ehm426. PMID: 17933755. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=35948999615&origin=inward.
Article9. Harn HJ, Lin SZ, Hung SH, Subeq YM, Li YS, Syu WS, Ding DC, Lee RP, Hsieh DK, Lin PC, Chiou TW. 2012; Adipose-derived stem cells can abrogate chemical-induced liver fibrosis and facilitate recovery of liver function. Cell Transplant. 21:2753–2764. DOI: 10.3727/096368912X652959. PMID: 22776464. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84879451028&origin=inward.
Article10. Lee EY, Xia Y, Kim WS, Kim MH, Kim TH, Kim KJ, Park BS, Sung JH. 2009; Hypoxia-enhanced wound-healing function of adipose-derived stem cells: increase in stem cell proliferation and up-regulation of VEGF and bFGF. Wound Repair Regen. 17:540–547. DOI: 10.1111/j.1524-475X.2009.00499.x. PMID: 19614919. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=67651171577&origin=inward.
Article11. Alexeev V, Arita M, Donahue A, Bonaldo P, Chu ML, Igoucheva O. 2014; Human adipose-derived stem cell transplantation as a potential therapy for collagen VI-related congenital muscular dystrophy. Stem Cell Res Ther. 5:21. DOI: 10.1186/scrt411. PMID: 24522088. PMCID: PMC4054951. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84894273668&origin=inward.
Article12. Gonzalez-Rey E, Gonzalez MA, Varela N, O'Valle F, Hernandez-Cortes P, Rico L, Büscher D, Delgado M. 2010; Human adipose-derived mesenchymal stem cells reduce inflammatory and T cell responses and induce regulatory T cells in vitro in rheumatoid arthritis. Ann Rheum Dis. 69:241–248. DOI: 10.1136/ard.2008.101881. PMID: 19124525. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=73449133395&origin=inward.
Article13. González MA, Gonzalez-Rey E, Rico L, Büscher D, Delgado M. 2009; Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology. 136:978–989. DOI: 10.1053/j.gastro.2008.11.041. PMID: 19135996. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=60449100851&origin=inward.
Article14. Yoon W, Jang S, Kook K. 2016; Use of adipose derived stem cells (ASCs) in treating autoimmune skin diseases: pilot study. Cytotherapy. 18(6 Suppl):S132–S133. https://www.isct-cytotherapy.org/article/S1465-3249(16)30283-3/fulltext. DOI: 10.1016/j.jcyt.2016.03.259.
Article15. Shin TH, Lee BC, Choi SW, Shin JH, Kang I, Lee JY, Kim JJ, Lee HK, Jung JE, Choi YW, Lee SH, Yoon JS, Choi JS, Lee CS, Seo Y, Kim HS, Kang KS. 2017; Human adipose tissue-derived mesenchymal stem cells alleviate atopic dermatitis via regulation of B lymphocyte maturation. Oncotarget. 8:512–522. DOI: 10.18632/oncotarget.13473. PMID: 27888809. PMCID: PMC5352174. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85009724082&origin=inward.
Article16. Back SK, Jeong KY, Li C, Lee J, Lee SB, Na HS. 2012; Chronically relapsing pruritic dermatitis in the rats treated as neonate with capsaicin; a potential rat model of human atopic dermatitis. J Dermatol Sci. 67:111–119. DOI: 10.1016/j.jdermsci.2012.05.006. PMID: 22721998. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84863877657&origin=inward.
Article17. Han RT, Kim HY, Ryu H, Jang W, Cha SH, Kim HY, Lee J, Back SK, Kim HJ, Na HS. 2018; Glyoxal-induced exacerbation of pruritus and dermatitis is associated with staphylococcus aureus colonization in the skin of a rat model of atopic dermatitis. J Dermatol Sci. 90:276–283. DOI: 10.1016/j.jdermsci.2018.02.012. PMID: 29496360. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85042416284&origin=inward.
Article18. Bunnell BA, Flaat M, Gagliardi C, Patel B, Ripoll C. 2008; Adipose-derived stem cells: isolation, expansion and differentiation. Methods. 45:115–120. DOI: 10.1016/j.ymeth.2008.03.006. PMID: 18593609. PMCID: PMC3668445. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=45849112916&origin=inward.
Article19. Yardeni T, Eckhaus M, Morris HD, Huizing M, Hoogstraten-Miller S. 2011; Retro-orbital injections in mice. Lab Anim (NY). 40:155–160. DOI: 10.1038/laban0511-155. PMID: 21508954. PMCID: PMC3158461. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=79955120806&origin=inward.
Article20. Lindner B, Burkard T, Schuler M. 2020; Phagocytosis assays with different pH-sensitive fluorescent particles and various readouts. Biotechniques. 68:245–250. DOI: 10.2144/btn-2020-0003. PMID: 32079414. PMID: 92657ebfb4e0438a859b8012786c9480. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85085231852&origin=inward.
Article21. Miksa M, Komura H, Wu R, Shah KG, Wang P. 2009; A novel method to determine the engulfment of apoptotic cells by macrophages using pHrodo succinimidyl ester. J Immunol Methods. 342:71–77. DOI: 10.1016/j.jim.2008.11.019. PMID: 19135446. PMCID: PMC2675277. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=59949083330&origin=inward.
Article22. Lecoeur H, Ledru E, Prévost MC, Gougeon ML. 1997; Strategies for phenotyping apoptotic peripheral human lymphocytes comparing ISNT, annexin-V and 7-AAD cytofluorometric staining methods. J Immunol Methods. 209:111–123. DOI: 10.1016/S0022-1759(97)00138-5. PMID: 9461328. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0031440380&origin=inward.
Article23. Kuraitis D, Williams L. 2018; Decolonization of Staphylococcus aureus in healthcare: a dermatology perspective. J Healthc Eng. 2018:2382050. DOI: 10.1155/2018/2382050. PMID: 30675332. PMCID: PMC6323510. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85059935545&origin=inward.24. Sohn MH, Kim JW, Kim WK, Jang GC, Kim KE. 2003; Staphylococcal enterotoxin B upregulates Fas-mediated apoptosis of peripheral blood mononuclear cells in childhood atopic dermatitis. Scand J Immunol. 57:62–67. DOI: 10.1046/j.1365-3083.2003.01183.x. PMID: 12542799. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0346250194&origin=inward.
Article25. Akdis CA, Akdis M, Trautmann A, Blaser K. 2000; Immune regulation in atopic dermatitis. Curr Opin Immunol. 12:641–646. DOI: 10.1016/S0952-7915(00)00156-4. PMID: 11102766. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0033739005&origin=inward.
Article26. Yoshino T, Asada H, Sano S, Nakamura T, Itami S, Tamura M, Yoshikawa K. 2000; Impaired responses of peripheral blood mononuclear cells to staphylococcal superantigen in patients with severe atopic dermatitis: a role of T cell apoptosis. J Invest Dermatol. 114:281–288. DOI: 10.1046/j.1523-1747.2000.00878.x. PMID: 10651987. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0034046429&origin=inward.
Article27. Grassmé H, Jendrossek V, Gulbins E. 2001; Molecular mechanisms of bacteria induced apoptosis. Apoptosis. 6:441–445. DOI: 10.1023/A:1012485506972. PMID: 11595833. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0035179629&origin=inward.28. Bień K, Żmigrodzka M, Orłowski P, Fruba A, Szymański Ł, Stankiewicz W, Nowak Z, Malewski T, Krzyżowska M. 2017; Involvement of Fas/FasL pathway in the murine model of atopic dermatitis. Inflamm Res. 66:679–690. DOI: 10.1007/s00011-017-1049-z. PMID: 28434120. PMCID: PMC5501908. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85018827670&origin=inward.
Article29. Farley SM, Dotson AD, Purdy DE, Sundholm AJ, Schneider P, Magun BE, Iordanov MS. 2006; Fas ligand elicits a caspase-independent proinflammatory response in human keratinocytes: implications for dermatitis. J Invest Dermatol. 126:2438–2451. DOI: 10.1038/sj.jid.5700477. PMID: 16858424. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=33750074486&origin=inward.
Article30. Bommhardt U, Chang KC, Swanson PE, Wagner TH, Tinsley KW, Karl IE, Hotchkiss RS. 2004; Akt decreases lymphocyte apoptosis and improves survival in sepsis. J Immunol. 172:7583–7591. DOI: 10.4049/jimmunol.172.12.7583. PMID: 15187138. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=2942527148&origin=inward.
Article31. Abdullraheem YF, Alzolibani AA, Mahmoud KH, Korsni AH, Al-Harbi MH, Hassanin KM, Al-Dhubaibi MS. 2017; Clinical study and assessment of leukocyte phagocytic function in children with atopic dermatitis in Qassim region of Saudi Arabia. Int J Health Sci (Qassim). 11:3–7. PMID: 29085260. PMCID: PMC5654183.32. Kim J, Hematti P. 2009; Mesenchymal stem cell-educated macrophages: a novel type of alternatively activated macrophages. Exp Hematol. 37:1445–1453. DOI: 10.1016/j.exphem.2009.09.004. PMID: 19772890. PMCID: PMC2783735. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=70449527554&origin=inward.
Article33. Porcheray F, Viaud S, Rimaniol AC, Léone C, Samah B, Dereuddre-Bosquet N, Dormont D, Gras G. 2005; Macrophage activation switching: an asset for the resolution of inflammation. Clin Exp Immunol. 142:481–489. DOI: 10.1111/j.1365-2249.2005.02934.x. PMID: 16297160. PMCID: PMC1809537. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=28344449833&origin=inward.
Article34. Rőszer T. 2015; Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediators Inflamm. 2015:816460. DOI: 10.1155/2015/816460. PMID: 26089604. PMCID: PMC4452191. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84930958641&origin=inward.
Article35. Yin X, Pang C, Bai L, Zhang Y, Geng L. 2016; [Adipose-derived stem cells promote the polarization from M1 macrophages to M2 macrophages]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 32:332–338. Chinese. PMID: 26927552. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84979097081&origin=inward.36. Han SC, Koo DH, Kang NJ, Yoon WJ, Kang GJ, Kang HK, Yoo ES. 2015; Docosahexaenoic acid alleviates atopic dermatitis by generating Tregs and IL-10/TGF-β-modified macrophages via a TGF-β-dependent mechanism. J Invest Dermatol. 135:1556–1564. DOI: 10.1038/jid.2014.488. PMID: 25405323. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84938074943&origin=inward.
Article37. Nguyen LT, Haney EF, Vogel HJ. 2011; The expanding scope of antimicrobial peptide structures and their modes of action. Trends Biotechnol. 29:464–472. DOI: 10.1016/j.tibtech.2011.05.001. PMID: 21680034. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=80051785173&origin=inward.
Article38. Mallbris L, Carlén L, Wei T, Heilborn J, Nilsson MF, Granath F, Ståhle M. 2010; Injury downregulates the expression of the human cathelicidin protein hCAP18/LL-37 in atopic dermatitis. Exp Dermatol. 19:442–449. DOI: 10.1111/j.1600-0625.2009.00918.x. PMID: 19645825. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=77953148855&origin=inward.
Article39. Nakatsuji T, Gallo RL. 2019; The role of the skin microbiome in atopic dermatitis. Ann Allergy Asthma Immunol. 122:263–269. Erratum in: Ann Allergy Asthma Immunol. 2019;123:529. DOI: 10.1016/j.anai.2018.12.003. PMID: 30550810. PMCID: PMC7147826. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85059450365&origin=inward.
Article40. Lee HJ, Jung M, Kim JH, Yoon NY, Choi EH. 2012; The effect of adipose-derived stem cell-cultured media on oxazolone treated atopic dermatitis-like murine model. Ann Dermatol. 24:181–188. DOI: 10.5021/ad.2012.24.2.181. PMID: 22577269. PMCID: PMC3346909. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84860625144&origin=inward.
Article41. Qian J, Hu Y, Zhao L, Xia J, Li C, Shi L, Xu F. 2016; Protective role of adipose-derived stem cells in Staphylococcus aureus-induced lung injury is mediated by RegIIIγ secretion. Stem Cells. 34:1947–1956. Erratum in: Stem Cells. 2016;34:2798. DOI: 10.1002/stem.2337. PMID: 26866937. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84979079592&origin=inward.
Article42. Geoghegan JA, Irvine AD, Foster TJ. 2018; Staphylococcus aureus and atopic dermatitis: a complex and evolving relationship. Trends Microbiol. 26:484–497. DOI: 10.1016/j.tim.2017.11.008. PMID: 29233606. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85037613425&origin=inward.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Staphylococcal Toxic Shock Syndrome Toxin-1 on Secretion of Interleukins and Interferon-gamma by Peripheral Blood Mononuclear Cells from Atopic Donors
- Concise Review: Differentiation of Human Adult Stem Cells Into Hepatocyte-like Cells In vitro
- Therapeutic Angiogenesis with Somatic Stem Cell Transplantation
- Response of Peripheral Blood Mononuclear Cells to Staphylococcus Aureus Exotoxin in Nasal Polyposis
- The Effect of Adipose-Derived Stem Cell-Cultured Media on Oxazolone Treated Atopic Dermatitis-Like Murine Model