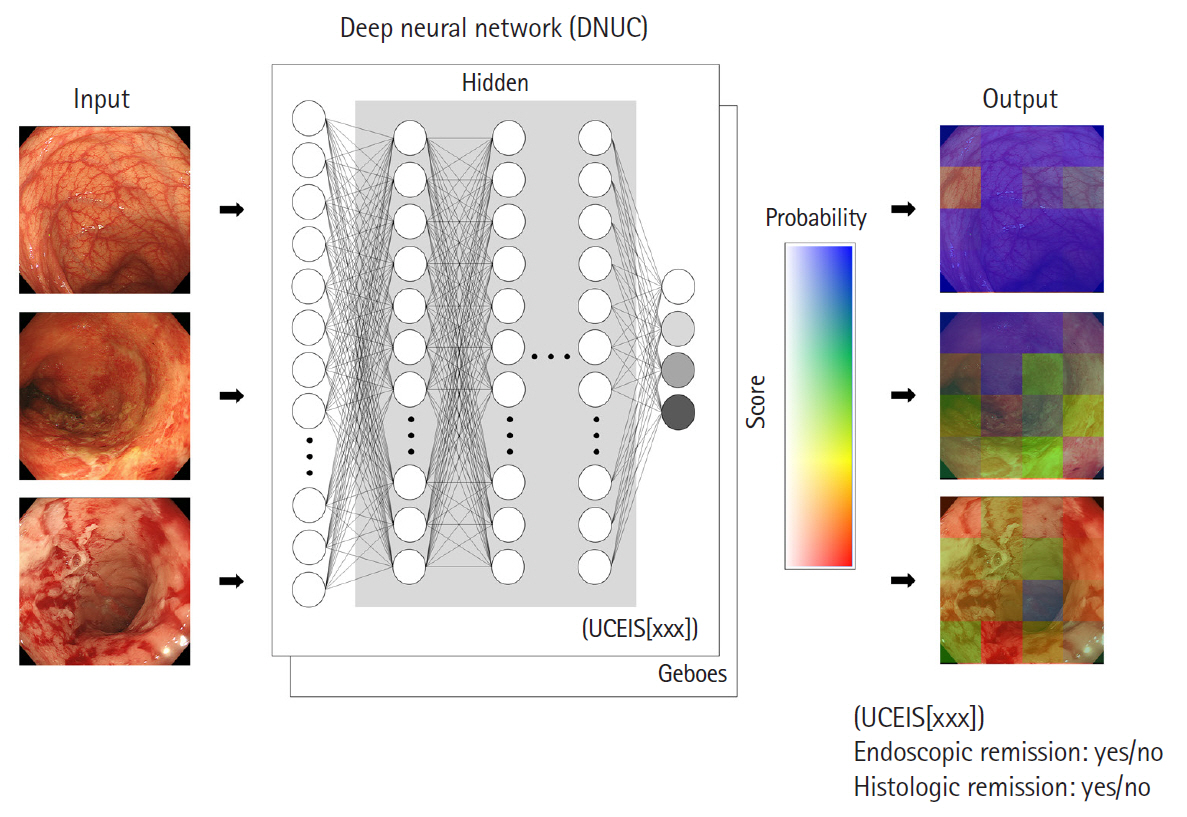
Intest Res.
2022 Apr;20(2):165-170. 10.5217/ir.2021.00079.
Artificial intelligence for endoscopy in inflammatory bowel disease
- Affiliations
-
- 1Department of Gastroenterology and Hepatology, Tokyo Medical and Dental University, Tokyo, Japan
- 2TMDU Advanced Research Institute, Tokyo Medical and Dental University, Tokyo, Japan
- 3Endoscopic Unit, Tokyo Medical and Dental University, Tokyo, Japan
- KMID: 2529562
- DOI: http://doi.org/10.5217/ir.2021.00079
Abstract
- Inflammatory bowel disease (IBD), with its 2 subtypes, Crohn’s disease and ulcerative colitis, is a complex chronic condition. A precise definition of disease activity and appropriate drug management greatly improve the clinical course while minimizing the risk or cost. Artificial intelligence (AI) has been used in several medical diseases or situations. Herein, we provide an overview of AI for endoscopy in IBD. We discuss how AI can improve clinical practice and how some components have already begun to shape our knowledge. There may be a time when we can use AI in clinical practice. As AI systems contribute to the exact diagnosis and treatment of human disease, we should continue to learn best practices in health care in the field of IBD.
Keyword
Figure
Cited by 1 articles
-
Endoscopic findings of immune checkpoint inhibitor-related gastrointestinal adverse events
Min Kyu Kim, Sung Wook Hwang
Clin Endosc. 2024;57(6):725-734. doi: 10.5946/ce.2024.003.
Reference
-
1. Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: an update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021; 160:1570–1583.
Article2. Feagan BG, Sandborn WJ, D’Haens G, et al. The role of centralized reading of endoscopy in a randomized controlled trial of mesalamine for ulcerative colitis. Gastroenterology. 2013; 145:149–157.
Article3. Osada T, Ohkusa T, Yokoyama T, et al. Comparison of several activity indices for the evaluation of endoscopic activity in UC: inter- and intraobserver consistency. Inflamm Bowel Dis. 2010; 16:192–197.
Article4. Kaneshiro M, Takenaka K, Suzuki K, et al. Pancolonic endoscopic and histologic evaluation for relapse prediction in patients with ulcerative colitis in clinical remission. Aliment Pharmacol Ther. 2021; 53:900–907.5. Cushing KC, Tan W, Alpers DH, Deshpande V, Ananthakrishnan AN. Complete histologic normalisation is associated with reduced risk of relapse among patients with ulcerative colitis in complete endoscopic remission. Aliment Pharmacol Ther. 2020; 51:347–355.
Article6. Ozawa T, Ishihara S, Fujishiro M, et al. Novel computer-assisted diagnosis system for endoscopic disease activity in patients with ulcerative colitis. Gastrointest Endosc. 2019; 89:416–421.
Article7. Higgins PD, Schwartz M, Mapili J, Krokos I, Leung J, Zimmermann EM. Patient defined dichotomous end points for remission and clinical improvement in ulcerative colitis. Gut. 2005; 54:782–788.
Article8. Maeda Y, Kudo SE, Mori Y, et al. Fully automated diagnostic system with artificial intelligence using endocytoscopy to identify the presence of histologic inflammation associated with ulcerative colitis (with video). Gastrointest Endosc. 2019; 89:408–415.
Article9. Geboes K, Riddell R, Ost A, Jensfelt B, Persson T, Löfberg R. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut. 2000; 47:404–409.
Article10. Stidham RW, Liu W, Bishu S, et al. Performance of a deep learning model vs human reviewers in grading endoscopic disease severity of patients with ulcerative colitis. JAMA Netw Open. 2019; 2:e193963.
Article11. Yao H, Najarian K, Gryak J, et al. Fully automated endoscopic disease activity assessment in ulcerative colitis. Gastrointest Endosc. 2021; 93:728–736.
Article12. Bossuyt P, Vermeire S, Bisschops R. Scoring endoscopic disease activity in IBD: artificial intelligence sees more and better than we do. Gut. 2020; 69:788–789.
Article13. Marchal-Bressenot A, Salleron J, Boulagnon-Rombi C, et al. Development and validation of the Nancy histological index for UC. Gut. 2017; 66:43–49.
Article14. Travis SP, Schnell D, Krzeski P, et al. Reliability and initial validation of the ulcerative colitis endoscopic index of severity. Gastroenterology. 2013; 145:987–995.
Article15. Takenaka K, Ohtsuka K, Fujii T, et al. Development and validation of a deep neural network for accurate evaluation of endoscopic images from patients with ulcerative colitis. Gastroenterology. 2020; 158:2150–2157.
Article16. Takenaka K, Ohtsuka K, Fujii T, Oshima S, Okamoto R, Watanabe M. Deep neural network accurately predicts prognosis of ulcerative colitis using endoscopic images. Gastroenterology. 2021; 160:2175–2177.
Article17. Gottlieb K, Requa J, Karnes W, et al. Central reading of ulcerative colitis clinical trial videos using neural networks. Gastroenterology. 2021; 160:710–719.
Article18. Takenaka K, Ohtsuka K, Kitazume Y, et al. Comparison of magnetic resonance and balloon enteroscopic examination of the small intestine in patients with Crohn’s disease. Gastroenterology. 2014; 147:334–342.
Article19. Enns RA, Hookey L, Armstrong D, et al. Clinical practice guidelines for the use of video capsule endoscopy. Gastroenterology. 2017; 152:497–514.
Article20. Sturm A, Maaser C, Calabrese E, et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 2: IBD scores and general principles and technical aspects. J Crohns Colitis. 2019; 13:273–284.
Article21. Ben-Horin S, Lahat A, Amitai MM, et al. Assessment of small bowel mucosal healing by video capsule endoscopy for the prediction of short-term and long-term risk of Crohn’s disease flare: a prospective cohort study. Lancet Gastroenterol Hepatol. 2019; 4:519–528.
Article22. Klang E, Barash Y, Margalit RY, et al. Deep learning algorithms for automated detection of Crohn’s disease ulcers by video capsule endoscopy. Gastrointest Endosc. 2020; 91:606–613.
Article23. Klang E, Grinman A, Soffer S, et al. Automated detection of Crohn’s disease intestinal strictures on capsule endoscopy images using deep neural networks. J Crohns Colitis. 2021; 15:749–756.
Article24. Barash Y, Azaria L, Soffer S, et al. Ulcer severity grading in video capsule images of patients with Crohn’s disease: an ordinal neural network solution. Gastrointest Endosc. 2021; 93:187–192.
Article25. Ding Z, Shi H, Zhang H, et al. Gastroenterologist-level identification of small-bowel diseases and normal variants by capsule endoscopy using a deep-learning model. Gastroenterology. 2019; 157:1044–1054.
Article26. Colombel JF, Rutgeerts P, Reinisch W, et al. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology. 2011; 141:1194–1201.
Article27. Hébuterne X, Lémann M, Bouhnik Y, et al. Endoscopic improvement of mucosal lesions in patients with moderate to severe ileocolonic Crohn’s disease following treatment with certolizumab pegol. Gut. 2013; 62:201–208.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Artificial intelligence in inflammatory bowel disease: implications for clinical practice and future directions
- The Future of Capsule Endoscopy: The Role of Artificial Intelligence and Other Technical Advancements
- Recent developments in small bowel endoscopy: the “black box” is now open!
- Role of artificial intelligence in diagnosing Barrett’s esophagus-related neoplasia
- Application of artificial intelligence for diagnosis of early gastric cancer based on magnifying endoscopy with narrow-band imaging