Kosin Med J.
2022 Mar;37(1):52-60. 10.7180/kmj.21.040.
Transcriptome analysis of the pathogenic ciliate Miamiensis avidus after hydrogen peroxide treatment
- Affiliations
-
- 1Department of Parasitology and Genetics, Institute for Medical Science, Kosin University College of Medicine, Busan, Korea
- KMID: 2527695
- DOI: http://doi.org/10.7180/kmj.21.040
Abstract
- Background
The scuticociliate Miamiensis avidus is a highly pathogenic ciliate responsible for serious damage to various organs of aquaculture fish. In particular, the olive flounder aquaculture industry is suffering massive losses due to M. avidus infection. Hydrogen peroxide (H2O2) is one of the most widely used chemicals for scuticociliate treatment. Despite the superior killing effect of H2O2, studies on transcription levels and gene expression changes after H2O2 treatment are limited. We conducted an mRNA transcriptome analysis to compare the expressed gene (DEG) profiles between the ciliate and cyst-like stages of M. avidus after H2O2 treatment.
Methods
We applied differentially expressed gene profiling to identify DEGs during the ciliate and cyst-like stages of M. avidus.
Results
There were 5,967 DEGs among the 9,075 transcripts identified, and 50 of these DEGs were significantly different (p<0.05). Among these, 21 DEGs were upregulated and 29 were downregulated in the cyst-like stage. The most significantly upregulated genes during the change to the cyst-like stage were cytochrome c oxidase genes. Genes related to the calcium channel were also highly upregulated.
Conclusion
The significant upregulation of cytochrome c gene expression and cytosolic calcium ion channel-related gene expression after H2O2 treatment suggests that ciliate mortality occurred through apoptosis. The formation of the cyst-like stage is considered a temporary form during the process of apoptosis. Information on the gene expression profile of M. avidus in response to H2O2 is expected to contribute to the understanding of the mechanism of action of therapeutic agents against this pathogen.
Keyword
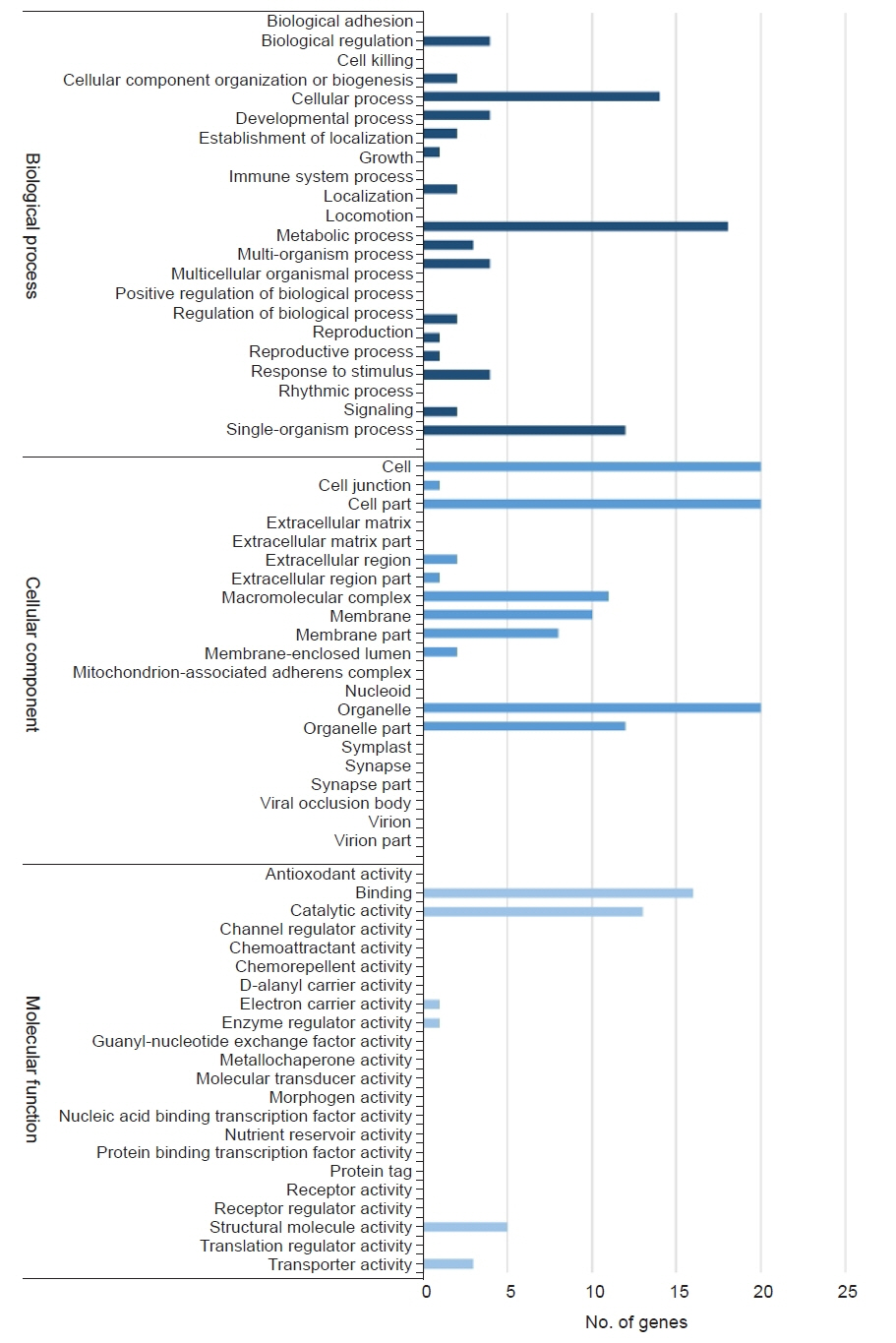
Figure
Reference
-
References
1. Song JY, Kitamura S, Oh MJ, Kang HS, Lee JH, Tanaka SJ, et al. Pathogenicity of Miamiensis avidus (syn. Philasterides dicentrarchi), Pseudocohnilembus persalinus, Pseudocohnilembus hargisi and Uronema marinum (Ciliophora, Scuticociliatida). Dis Aquat Organ. 2009; 83:133–43.
Article2. Retallack H, Okihiro MS, Britton E, Sommeran SV, DeRisi JL. Metagenomic next-generation sequencing reveals Miamiensis avidus (Ciliophora: Scuticociliatida) in the 2017 epizootic of leopard sharks (Triakis semifasciata) in San Francisco Bay, California, USA. J Wildl Dis. 2019; 55:375–86.
Article3. Proto WR, Coombs GH, Mottram JC. Cell death in parasitic protozoa: regulated or incidental? Nat Rev Microbiol. 2013; 11:58–66.
Article4. Gechev TS, Hille J. Hydrogen peroxide as a signal controlling plant programmed cell death. J Cell Biol. 2005; 168:17–20.
Article5. Lee JH, Park JJ, Choi JH, Shin DH, Park KH. Anti-scuticociliate effects of aquatic hydrogen peroxide preparation in olive flounder Paralichthys olivaceus. J Fish Pathol. 2017; 30:107–14.6. U.S. Food and Drug Administration. CFR - Code of Federal Regulations Title 21 [Internet]. Silver Spring: U.S. Food and Drug Administration;c2022. [cited 2021 Dec 15]. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=530.7. Crosbie PB, Munday BL. Environmental factors and chemical agents affecting the growth of the pathogenic marine ciliate Uronema nigricans. Dis Aquat Organ. 1999; 36:213–9.
Article8. Jee BY, Jo MR, Kim JW, Park MS. In vitro efficacy of formalin, hydrogen peroxide and copper sulfate on the scuticocilliate Uronema marinum at low salinity. J Fish Pathol. 2002; 15:111–5.9. Whang I, Kang HS, Lee J. Identification of scuticociliates (Pseudocohnilembus persalinus, P. longisetus, Uronema marinum and Miamiensis avidus) based on the cox1 sequence. Parasitol Int. 2013; 62:7–13.
Article10. Gebler GF. Phylogenetic relationship among polymorphic Oligohymenophorean ciliates, with gene expression in life-history stages of Miamiensis avidus (Ciliophora, Oligohymenophorea) [dissertation]. College Park: University of Maryland;2007.11. Sogame Y, Kojima K, Takeshita T, Kinoshita E, Matsuoka T. Identification of differentially expressed water-insoluble proteins in the encystment process of Colpoda cucullus by two-dimensional electrophoresis and LC-MS/MS analysis. J Eukaryot Microbiol. 2014; 61:51–60.
Article12. Villalobo E, Moch C, Perasso R, Baroin-Tourancheau A. Searching for excystment-regulated genes in Sterkiella histriomuscorum (Ciliophora, Oxytrichidae): a mRNA differential display analysis of gene expression in excysting cells. J Eukaryot Microbiol. 2001; 48:382–90.
Article13. Matsuoka T. Early signaling pathways mediating dormant cyst formation in terrestrial unicellular eukaryote Colpoda. FEMS Microbiol Lett. 2021; 368:fnab019.14. Plattner H, Klauke N. Calcium in ciliated protozoa: sources, regulation, and calcium-regulated cell functions. Int Rev Cytol. 2001; 201:115–208.
Article15. Shindo T, Van der Hoorn RA. Papain-like cysteine proteases: key players at molecular battlefields employed by both plants and their invaders. Mol Plant Pathol. 2008; 9:119–25.
Article16. Misas-Villamil JC, van der Hoorn RA, Doehlemann G. Papain-like cysteine proteases as hubs in plant immunity. New Phytol. 2016; 212:902–7.
Article17. Gannavaram S, Debrabant A. Programmed cell death in Leishmania: biochemical evidence and role in parasite infectivity. Front Cell Infect Microbiol. 2012; 2:95.
Article18. Welburn SC, Macleod E, Figarella K, Duzensko M. Programmed cell death in African trypanosomes. Parasitology. 2006; 132 Suppl:S7–18.
Article19. Stowe DF, Camara AK. Mitochondrial reactive oxygen species production in excitable cells: modulators of mitochondrial and cell function. Antioxid Redox Signal. 2009; 11:1373–414.
Article20. Wang C, Youle RJ. The role of mitochondria in apoptosis*. Annu Rev Genet. 2009; 43:95–118.
Article21. Ghosh AS, Dutta S, Raha S. Hydrogen peroxide-induced apoptosis-like cell death in Entamoeba histolytica. Parasitol Int. 2010; 59:166–72.
Article22. Kundu-Michalik S, Bisotti MA, Lipsius E, Bauche A, Kruppa A, Klokow T, et al. Nucleolar binding sequences of the ribosomal protein S6e family reside in evolutionary highly conserved peptide clusters. Mol Biol Evol. 2008; 25:580–90.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Identification of the transcriptome profile of Miamiensis avidus after mebendazole treatment
- The Effects of Hydrogen Peroxide on the Migration and Proliferation of the Human Keratinocytes during Wound Healing
- Hydrogen Peroxide as an Effective Disinfectant for Pasteurella multocida
- A case of hydrogen peroxide-induced proctocolitis in a child
- A Case of Hydrogen Peroxide Induced Proctocolitis