Int J Stem Cells.
2022 Feb;15(1):41-59. 10.15283/ijsc22004.
Engineering Brain Organoids: Toward Mature Neural Circuitry with an Intact Cytoarchitecture
- Affiliations
-
- 1Department of Biological Sciences, Korea Advanced Institute of Science and Technology (KAIST), Daejeon, Korea
- 2KAIST-Wonjin Cell Therapy Center, Korea Advanced Institute of Science and Technology (KAIST), Daejeon, Korea
- KMID: 2526737
- DOI: http://doi.org/10.15283/ijsc22004
Abstract
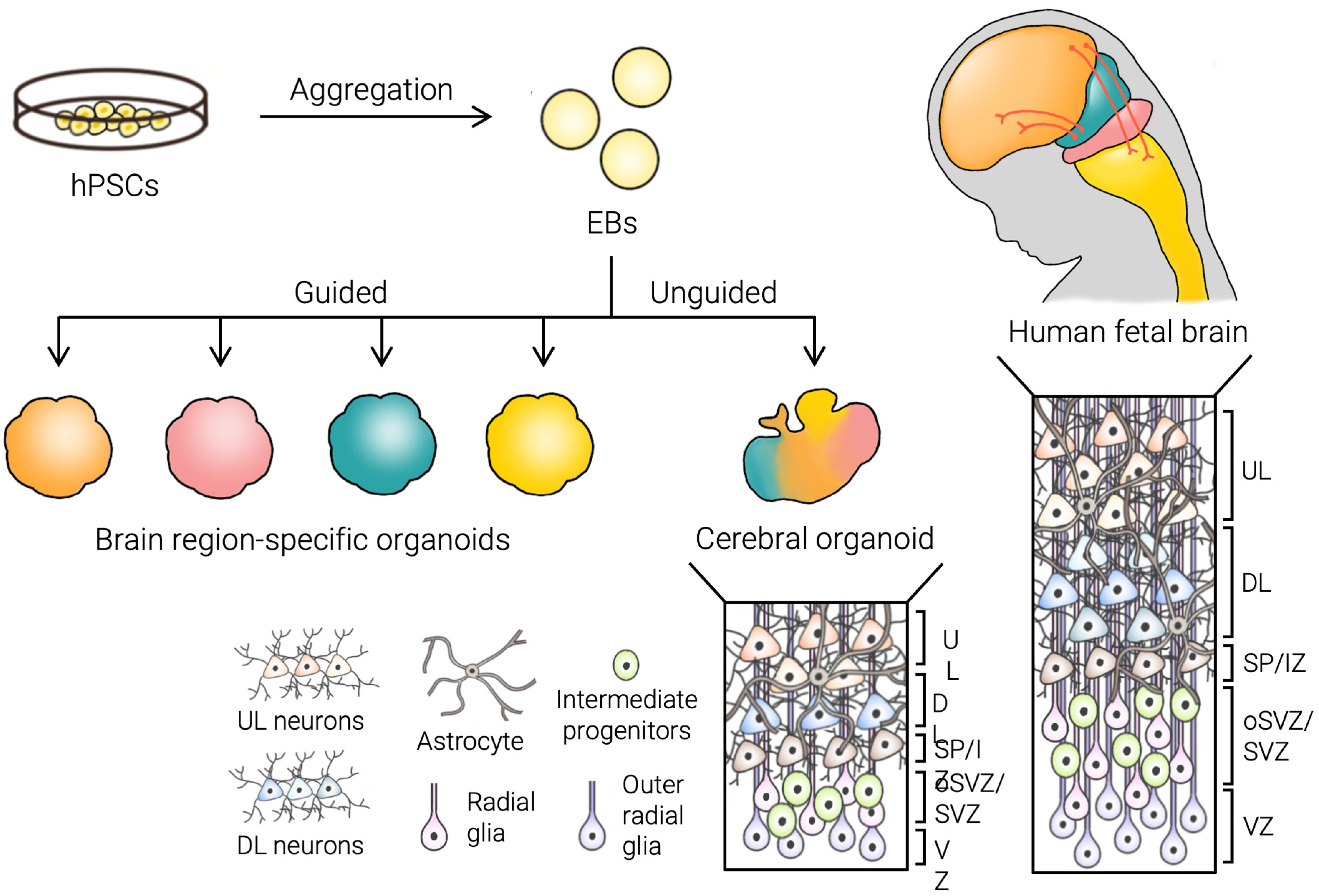
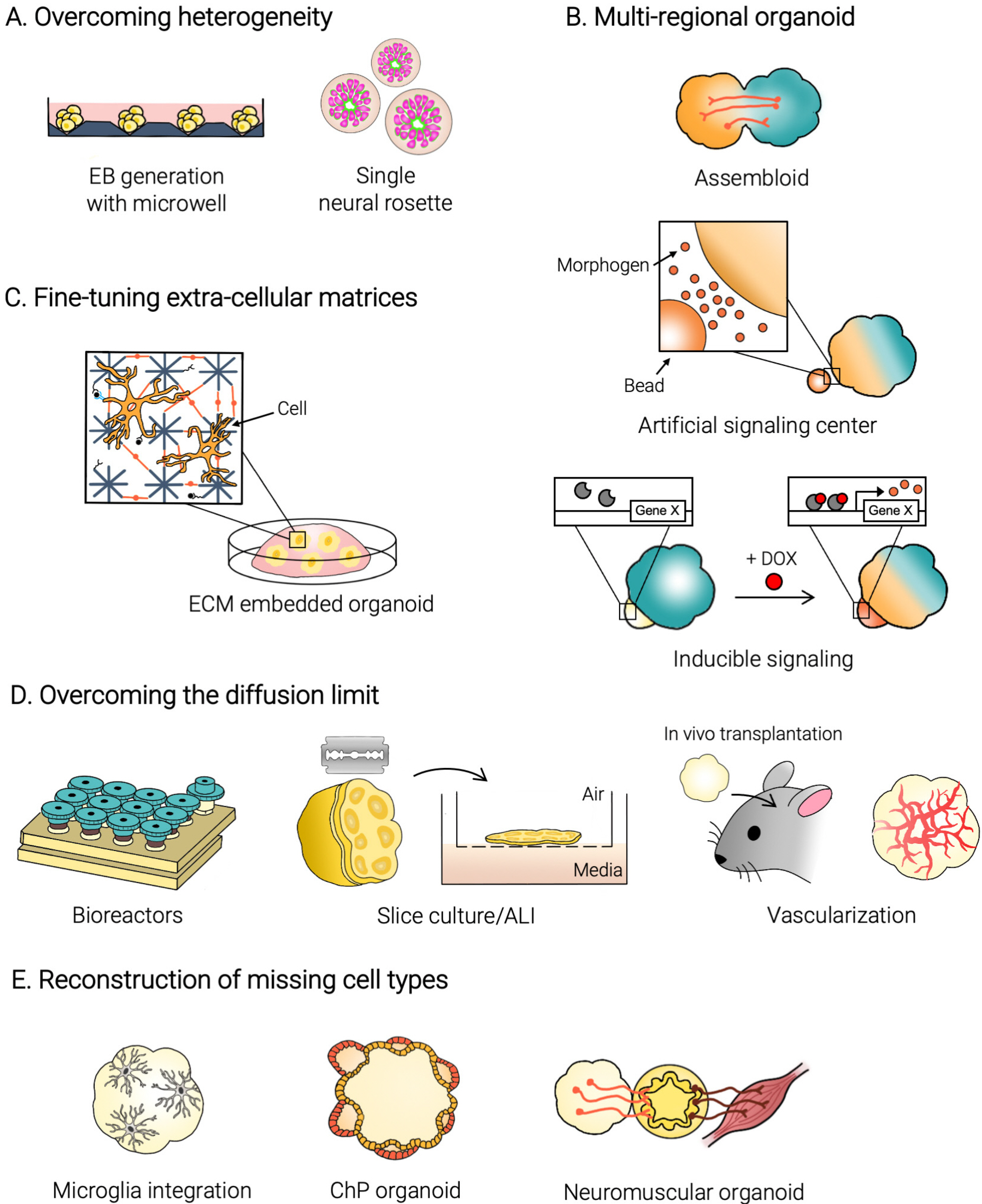
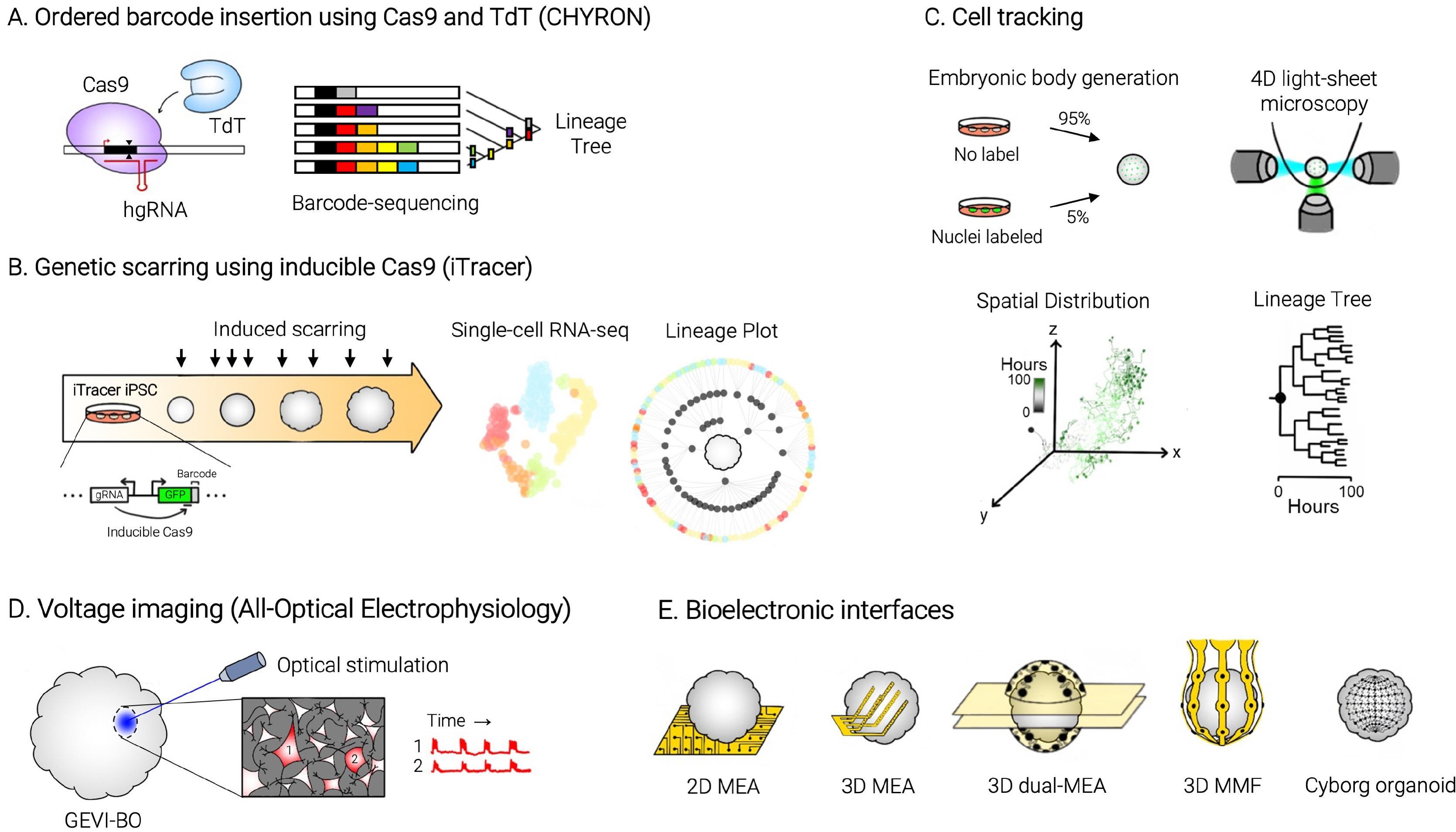
- The emergence of brain organoids as a model system has been a tremendously exciting development in the field of neuroscience. Brain organoids are a gateway to exploring the intricacies of human-specific neurogenesis that have so far eluded the neuroscience community. Regardless, current culture methods have a long way to go in terms of accuracy and reproducibility. To perfectly mimic the human brain, we need to recapitulate the complex in vivo context of the human fetal brain and achieve mature neural circuitry with an intact cytoarchitecture. In this review, we explore the major challenges facing the current brain organoid systems, potential technical breakthroughs to advance brain organoid techniques up to levels similar to an in vivo human developing brain, and the future prospects of this technology.
Keyword
Figure
Cited by 2 articles
-
Lo and Behold, the Lab-Grown Organs Have Arrived!
Jaesang Kim
Int J Stem Cells. 2022;15(1):1-2. doi: 10.15283/ijsc22026.Transcriptional Signature of Valproic Acid-Induced Neural Tube Defects in Human Spinal Cord Organoids
Ju-Hyun Lee, Mohammed R. Shaker, Si-Hyung Park, Woong Sun
Int J Stem Cells. 2023;16(4):385-393. doi: 10.15283/ijsc23012.
Reference
-
References
1. Lui JH, Hansen DV, Kriegstein AR. 2011; Development and evolution of the human neocortex. Cell. 146:18–36. Erratum in: Cell 2011;146:332. DOI: 10.1016/j.cell.2011.06.030. PMCID: PMC3610574. PMID: 21729779.
Article2. Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. 1998; Embryonic stem cell lines derived from human blastocysts. Science. 282:1145–1147. Erratum in: Science 1998;282:1827. DOI: 10.1126/science.282.5391.1145. PMID: 9804556.
Article3. Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. 2007; Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 131:861–872. DOI: 10.1016/j.cell.2007.11.019. PMID: 18035408.
Article4. Eiraku M, Watanabe K, Matsuo-Takasaki M, Kawada M, Yonemura S, Matsumura M, Wataya T, Nishiyama A, Muguruma K, Sasai Y. 2008; Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell. 3:519–532. DOI: 10.1016/j.stem.2008.09.002. PMID: 18983967.
Article5. Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA. 2013; Cerebral organoids model human brain development and microcephaly. Nature. 501:373–379. DOI: 10.1038/nature12517. PMID: 23995685. PMCID: PMC3817409.
Article6. Lancaster MA, Knoblich JA. 2014; Generation of cerebral organoids from human pluripotent stem cells. Nat Protoc. 9:2329–2340. DOI: 10.1038/nprot.2014.158. PMID: 25188634. PMCID: PMC4160653.
Article7. Qian X, Nguyen HN, Song MM, Hadiono C, Ogden SC, Hammack C, Yao B, Hamersky GR, Jacob F, Zhong C, Yoon KJ, Jeang W, Lin L, Li Y, Thakor J, Berg DA, Zhang C, Kang E, Chickering M, Nauen D, Ho CY, Wen Z, Christian KM, Shi PY, Maher BJ, Wu H, Jin P, Tang H, Song H, Ming GL. 2016; Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell. 165:1238–1254. DOI: 10.1016/j.cell.2016.04.032. PMID: 27118425. PMCID: PMC4900885.
Article8. Sakaguchi H, Kadoshima T, Soen M, Narii N, Ishida Y, Ohgushi M, Takahashi J, Eiraku M, Sasai Y. 2015; Generation of functional hippocampal neurons from self-organizing human embryonic stem cell-derived dorsomedial telencephalic tissue. Nat Commun. 6:8896. DOI: 10.1038/ncomms9896. PMID: 26573335. PMCID: PMC4660208.
Article9. Muguruma K, Nishiyama A, Kawakami H, Hashimoto K, Sasai Y. 2015; Self-organization of polarized cerebellar tissue in 3D culture of human pluripotent stem cells. Cell Rep. 10:537–550. DOI: 10.1016/j.celrep.2014.12.051. PMID: 25640179.
Article10. Di Lullo E, Kriegstein AR. 2017; The use of brain organoids to investigate neural development and disease. Nat Rev Neurosci. 18:573–584. DOI: 10.1038/nrn.2017.107. PMID: 28878372. PMCID: PMC5667942.
Article11. Gordon A, Yoon SJ, Tran SS, Makinson CD, Park JY, Andersen J, Valencia AM, Horvath S, Xiao X, Huguenard JR, Pașca SP, Geschwind DH. 2021; Long-term maturation of human cortical organoids matches key early postnatal transitions. Nat Neurosci. 24:331–342. DOI: 10.1038/s41593-021-00802-y. PMID: 33619405. PMCID: PMC8109149.
Article12. Bhaduri A, Andrews MG, Mancia Leon W, Jung D, Shin D, Allen D, Jung D, Schmunk G, Haeussler M, Salma J, Pollen AA, Nowakowski TJ, Kriegstein AR. 2020; Cell stress in cortical organoids impairs molecular subtype specification. Nature. 578:142–148. DOI: 10.1038/s41586-020-1962-0. PMID: 31996853. PMCID: PMC7433012.
Article13. Quadrato G, Nguyen T, Macosko EZ, Sherwood JL, Min Yang S, Berger DR, Maria N, Scholvin J, Goldman M, Kinney JP, Boyden ES, Lichtman JW, Williams ZM, McCarroll SA, Arlotta P. 2017; Cell diversity and network dynamics in photosensitive human brain organoids. Nature. 545:48–53. DOI: 10.1038/nature22047. PMID: 28445462. PMCID: PMC5659341.
Article14. Tanaka Y, Cakir B, Xiang Y, Sullivan GJ, Park IH. 2020; Synthetic analyses of single-cell transcriptomes from multiple brain organoids and fetal brain. Cell Rep. 30:1682–1689.e3. DOI: 10.1016/j.celrep.2020.01.038. PMID: 32049002. PMCID: PMC7043376.
Article15. Lancaster MA, Corsini NS, Wolfinger S, Gustafson EH, Phillips AW, Burkard TR, Otani T, Livesey FJ, Knoblich JA. 2017; Guided self-organization and cortical plate formation in human brain organoids. Nat Biotechnol. 35:659–666. DOI: 10.1038/nbt.3906. PMID: 28562594. PMCID: PMC5824977.
Article16. Dong X, Xu SB, Chen X, Tao M, Tang XY, Fang KH, Xu M, Pan Y, Chen Y, He S, Liu Y. 2021; Human cerebral organoids establish subcortical projections in the mouse brain after transplantation. Mol Psychiatry. 26:2964–2976. DOI: 10.1038/s41380-020-00910-4. PMID: 33051604. PMCID: PMC8505255.
Article17. Choi YY, Chung BG, Lee DH, Khademhosseini A, Kim JH, Lee SH. 2010; Controlled-size embryoid body formation in concave microwell arrays. Biomaterials. 31:4296–4303. DOI: 10.1016/j.biomaterials.2010.01.115. PMID: 20206991.
Article18. Hwang YS, Chung BG, Ortmann D, Hattori N, Moeller HC, Khademhosseini A. 2009; Microwell-mediated control of embryoid body size regulates embryonic stem cell fate via differential expression of WNT5a and WNT11. Proc Natl Acad Sci U S A. 106:16978–16983. DOI: 10.1073/pnas.0905550106. PMID: 19805103. PMCID: PMC2761314.
Article19. Vrij EJ, Espinoza S, Heilig M, Kolew A, Schneider M, van Blitterswijk CA, Truckenmüller RK, Rivron NC. 2016; 3D high throughput screening and profiling of embryoid bodies in thermoformed microwell plates. Lab Chip. 16:734–742. DOI: 10.1039/C5LC01499A. PMID: 26775648.
Article20. Nelson CM, Vanduijn MM, Inman JL, Fletcher DA, Bissell MJ. 2006; Tissue geometry determines sites of mammary branching morphogenesis in organotypic cultures. Science. 314:298–300. DOI: 10.1126/science.1131000. PMID: 17038622. PMCID: PMC2933179.
Article21. Cerchiari A, Garbe JC, Todhunter ME, Jee NY, Pinney JR, LaBarge MA, Desai TA, Gartner ZJ. 2015; Formation of spatially and geometrically controlled three-dimensional tissues in soft gels by sacrificial micromolding. Tissue Eng Part C Methods. 21:541–547. DOI: 10.1089/ten.tec.2014.0450. PMID: 25351430. PMCID: PMC4442595.
Article22. O'Leary DD, Chou SJ, Sahara S. 2007; Area patterning of the mammalian cortex. Neuron. 56:252–269. DOI: 10.1016/j.neuron.2007.10.010. PMID: 17964244.23. Paşca AM, Sloan SA, Clarke LE, Tian Y, Makinson CD, Huber N, Kim CH, Park JY, O'Rourke NA, Nguyen KD, Smith SJ, Huguenard JR, Geschwind DH, Barres BA, Paşca SP. 2015; Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat Methods. 12:671–678. DOI: 10.1038/nmeth.3415. PMID: 26005811. PMCID: PMC4489980.
Article24. Birey F, Andersen J, Makinson CD, Islam S, Wei W, Huber N, Fan HC, Metzler KRC, Panagiotakos G, Thom N, O'Rourke NA, Steinmetz LM, Bernstein JA, Hallmayer J, Huguenard JR, Paşca SP. 2017; Assembly of functionally integrated human forebrain spheroids. Nature. 545:54–59. DOI: 10.1038/nature22330. PMID: 28445465. PMCID: PMC5805137.
Article25. Xiang Y, Tanaka Y, Patterson B, Kang YJ, Govindaiah G, Roselaar N, Cakir B, Kim KY, Lombroso AP, Hwang SM, Zhong M, Stanley EG, Elefanty AG, Naegele JR, Lee SH, Weissman SM, Park IH. 2017; Fusion of regionally specified hPSC-derived organoids models human brain development and interneuron migration. Cell Stem Cell. 21:383–398.e7. DOI: 10.1016/j.stem.2017.07.007. PMID: 28757360. PMCID: PMC5720381.
Article26. Jo J, Xiao Y, Sun AX, Cukuroglu E, Tran HD, Göke J, Tan ZY, Saw TY, Tan CP, Lokman H, Lee Y, Kim D, Ko HS, Kim SO, Park JH, Cho NJ, Hyde TM, Kleinman JE, Shin JH, Weinberger DR, Tan EK, Je HS, Ng HH. 2016; Midbrain-like organoids from human pluripotent stem cells contain functional dopaminergic and neuromelanin-producing neurons. Cell Stem Cell. 19:248–257. DOI: 10.1016/j.stem.2016.07.005. PMID: 27476966. PMCID: PMC5510242.
Article27. Miura Y, Li MY, Birey F, Ikeda K, Revah O, Thete MV, Park JY, Puno A, Lee SH, Porteus MH, Pașca SP. 2020; Generation of human striatal organoids and cortico-striatal assembloids from human pluripotent stem cells. Nat Biotechnol. 38:1421–1430. DOI: 10.1038/s41587-020-00763-w. PMID: 33273741.
Article28. Pellegrini L, Bonfio C, Chadwick J, Begum F, Skehel M, Lancaster MA. 2020; Human CNS barrier-forming organoids with cerebrospinal fluid production. Science. 369:eaaz5626. DOI: 10.1126/science.aaz5626. PMID: 32527923. PMCID: PMC7116154.
Article29. Xiang Y, Tanaka Y, Cakir B, Patterson B, Kim KY, Sun P, Kang YJ, Zhong M, Liu X, Patra P, Lee SH, Weissman SM, Park IH. 2019; hESC-derived thalamic organoids form reciprocal projections when fused with cortical organoids. Cell Stem Cell. 24:487–497.e7. DOI: 10.1016/j.stem.2018.12.015. PMID: 30799279. PMCID: PMC6853597.
Article30. Valiulahi P, Vidyawan V, Puspita L, Oh Y, Juwono VB, Sittipo P, Friedlander G, Yahalomi D, Sohn JW, Lee YK, Yoon JK, Shim JW. 2021; Generation of caudal-type serotonin neurons and hindbrain-fate organoids from hPSCs. Stem Cell Reports. 16:1938–1952. DOI: 10.1016/j.stemcr.2021.06.006. PMID: 34242615. PMCID: PMC8365029.
Article31. Ogura T, Sakaguchi H, Miyamoto S, Takahashi J. 2018; Three-dimensional induction of dorsal, intermediate and ventral spinal cord tissues from human pluripotent stem cells. Development. 145:dev162214. DOI: 10.1242/dev.162214. PMID: 30061169. PMCID: PMC6124545.
Article32. Yoon SJ, Elahi LS, Pașca AM, Marton RM, Gordon A, Revah O, Miura Y, Walczak EM, Holdgate GM, Fan HC, Huguenard JR, Geschwind DH, Pașca SP. 2019; Reliability of human cortical organoid generation. Nat Methods. 16:75–78. DOI: 10.1038/s41592-018-0255-0. PMID: 30573846. PMCID: PMC6677388.
Article33. Velasco S, Kedaigle AJ, Simmons SK, Nash A, Rocha M, Quadrato G, Paulsen B, Nguyen L, Adiconis X, Regev A, Levin JZ, Arlotta P. 2019; Individual brain organoids reproducibly form cell diversity of the human cerebral cortex. Nature. 570:523–527. DOI: 10.1038/s41586-019-1289-x. PMID: 31168097. PMCID: PMC6906116.
Article34. Libby ARG, Joy DA, Elder NH, Bulger EA, Krakora MZ, Gaylord EA, Mendoza-Camacho F, Butts JC, McDevitt TC. 2021; Axial elongation of caudalized human organoids mimics aspects of neural tube development. Development. 148:dev198275. DOI: 10.1242/dev.198275. PMID: 34142711. PMCID: PMC8254868.
Article35. Gabriel E, Albanna W, Pasquini G, Ramani A, Josipovic N, Mariappan A, Schinzel F, Karch CM, Bao G, Gottardo M, Suren AA, Hescheler J, Nagel-Wolfrum K, Persico V, Rizzoli SO, Altmüller J, Riparbelli MG, Callaini G, Goureau O, Papantonis A, Busskamp V, Schneider T, Gopalakrishnan J. 2021; Human brain organoids assemble functionally integrated bilateral optic vesicles. Cell Stem Cell. 28:1740–1757.e8. DOI: 10.1016/j.stem.2021.07.010. PMID: 34407456.
Article36. Bagley JA, Reumann D, Bian S, Lévi-Strauss J, Knoblich JA. 2017; Fused cerebral organoids model interactions between brain regions. Nat Methods. 14:743–751. DOI: 10.1038/nmeth.4304. PMID: 28504681. PMCID: PMC5540177.
Article37. Kasai T, Suga H, Sakakibara M, Ozone C, Matsumoto R, Kano M, Mitsumoto K, Ogawa K, Kodani Y, Nagasaki H, Inoshita N, Sugiyama M, Onoue T, Tsunekawa T, Ito Y, Takagi H, Hagiwara D, Iwama S, Goto M, Banno R, Takahashi J, Arima H. 2020; Hypothalamic contribution to pituitary functions is recapitulated in vitro using 3D-cultured human iPS cells. Cell Rep. 30:18–24.e5. DOI: 10.1016/j.celrep.2019.12.009. PMID: 31914385.38. Andersen J, Revah O, Miura Y, Thom N, Amin ND, Kelley KW, Singh M, Chen X, Thete MV, Walczak EM, Vogel H, Fan HC, Paşca SP. 2020; Generation of functional human 3D cortico-motor assembloids. Cell. 183:1913–1929.e26. DOI: 10.1016/j.cell.2020.11.017. PMID: 33333020. PMCID: PMC8711252.
Article39. Sansom SN, Livesey FJ. 2009; Gradients in the brain: the control of the development of form and function in the cerebral cortex. Cold Spring Harb Perspect Biol. 1:a002519. DOI: 10.1101/cshperspect.a002519. PMID: 20066088. PMCID: PMC2742095.
Article40. Gurdon JB, Mitchell A, Mahony D. 1995; Direct and continuous assessment by cells of their position in a morphogen gradient. Nature. 376:520–521. DOI: 10.1038/376520a0. PMID: 7637784.
Article41. Ben-Reuven L, Reiner O. 2020; Toward spatial identities in human brain organoids-on-chip induced by morphogen-soaked beads. Bioengineering (Basel). 7:164. DOI: 10.3390/bioengineering7040164. PMID: 33352983. PMCID: PMC7766968. PMID: ff5862d4622e47668183887d00b1cf5f.
Article42. Xu PF, Borges RM, Fillatre J, de Oliveira-Melo M, Cheng T, Thisse B, Thisse C. 2021; Construction of a mammalian embryo model from stem cells organized by a morphogen signalling centre. Nat Commun. 12:3277. DOI: 10.1038/s41467-021-23653-4. PMID: 34078907. PMCID: PMC8172561. PMID: 0246bf48802949329082b7c4d63be793.
Article43. Glykofrydis F, Cachat E, Berzanskyte I, Dzierzak E, Davies JA. 2021; Bioengineering self-organizing signaling centers to control embryoid body pattern elaboration. ACS Synth Biol. 10:1465–1480. DOI: 10.1021/acssynbio.1c00060. PMID: 34019395.
Article44. Cederquist GY, Asciolla JJ, Tchieu J, Walsh RM, Cornacchia D, Resh MD, Studer L. 2019; Specification of positional identity in forebrain organoids. Nat Biotechnol. 37:436–444. DOI: 10.1038/s41587-019-0085-3. PMID: 30936566. PMCID: PMC6447454.
Article45. Repina NA, Bao X, Zimmermann JA, Joy DA, Kane RS, Schaffer DV. 2019. Optogenetic control of Wnt signaling for modeling early embryogenic patterning with human pluripotent stem cells. bioRxiv 665695 [Preprint]. Available from: https://doi.org/10.1101/665695. cited 2019 Jun 10. DOI: 10.1101/665695. PMCID: PMC6706021.
Article46. Martínez-Ara G, Taberner N, Takayama M, Sandaltzopoulou E, Villava CE, Takata N, Eiraku M, Ebisuya M. 2021. Optogenetic control of apical constriction induces synthetic morphogenesis in mammalian tissues. bioRxiv 440475 [Preprint]. Available from: https://doi.org/10.1101/2021.04.20.440475. cited 2021 Apr 21. DOI: 10.1101/2021.04.20.440475. PMID: 34494114. PMCID: PMC8451065.
Article47. Legnini I, Emmenegger L, Wurmus R, Zappulo A, Martinez AO, Jara CC, Boltengagen A, Hessler T, Mastrobuoni G, Rybak-Wolf A, Kempa S, Zinzen R, Woehler A, Rajewsky N. 2021. Optogenetic perturbations of RNA expression in tissue space. bioRxiv 461850 [Preprint]. Available from: https://doi.org/10.1101/2021.09.26.461850. cited 2022 Feb 9. DOI: 10.1101/2021.09.26.461850. PMID: 33585804. PMCID: PMC7866843.
Article48. Nihongaki Y, Furuhata Y, Otabe T, Hasegawa S, Yoshimoto K, Sato M. 2017; CRISPR-Cas9-based photoactivatable transcription systems to induce neuronal differentiation. Nat Methods. 14:963–966. DOI: 10.1038/nmeth.4430. PMID: 28892089.
Article49. Rifes P, Isaksson M, Rathore GS, Aldrin-Kirk P, Møller OK, Barzaghi G, Lee J, Egerod KL, Rausch DM, Parmar M, Pers TH, Laurell T, Kirkeby A. 2020; Modeling neural tube development by differentiation of human embryonic stem cells in a microfluidic WNT gradient. Nat Biotechnol. 38:1265–1273. Erratum in: Nat Biotechnol 2020;38:1357. DOI: 10.1038/s41587-020-0525-0. PMID: 32451506.
Article50. Brown TE, Anseth KS. 2017; Spatiotemporal hydrogel biomaterials for regenerative medicine. Chem Soc Rev. 46:6532–6552. DOI: 10.1039/C7CS00445A. PMID: 28820527. PMCID: PMC5662487.
Article51. Zimmermann DR, Dours-Zimmermann MT. 2008; Extracellular matrix of the central nervous system: from neglect to challenge. Histochem Cell Biol. 130:635–653. DOI: 10.1007/s00418-008-0485-9. PMID: 18696101.
Article52. Barros CS, Franco SJ, Müller U. 2011; Extracellular matrix: functions in the nervous system. Cold Spring Harb Perspect Biol. 3:a005108. DOI: 10.1101/cshperspect.a005108. PMID: 21123393. PMCID: PMC3003458.
Article53. Long KR, Huttner WB. 2019; How the extracellular matrix shapes neural development. Open Biol. 9:180216. DOI: 10.1098/rsob.180216. PMID: 30958121. PMCID: PMC6367132. PMID: 1bc187ecda974d4e91226b2f146430b5.
Article54. Luo C, Lancaster MA, Castanon R, Nery JR, Knoblich JA, Ecker JR. 2016; Cerebral organoids recapitulate epigenomic signatures of the human fetal brain. Cell Rep. 17:3369–3384. DOI: 10.1016/j.celrep.2016.12.001. PMID: 28009303. PMCID: PMC5495578.
Article55. Bian S, Repic M, Guo Z, Kavirayani A, Burkard T, Bagley JA, Krauditsch C, Knoblich JA. 2018; Genetically engineered cerebral organoids model brain tumor formation. Nat Methods. 15:631–639. Erratum in: Nat Methods 2018; 15:748. DOI: 10.1038/s41592-018-0070-7. PMCID: PMC6071863. PMID: 30038414.
Article56. Hughes CS, Postovit LM, Lajoie GA. 2010; Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics. 10:1886–1890. DOI: 10.1002/pmic.200900758. PMID: 20162561.
Article57. Bandtlow CE, Zimmermann DR. 2000; Proteoglycans in the developing brain: new conceptual insights for old proteins. Physiol Rev. 80:1267–1290. DOI: 10.1152/physrev.2000.80.4.1267. PMID: 11015614.
Article58. Miyata S, Kitagawa H. 2017; Formation and remodeling of the brain extracellular matrix in neural plasticity: roles of chondroitin sulfate and hyaluronan. Biochim Biophys Acta Gen Subj. 1861:2420–2434. DOI: 10.1016/j.bbagen.2017.06.010. PMID: 28625420.
Article59. Ma W, Tavakoli T, Derby E, Serebryakova Y, Rao MS, Mattson MP. 2008; Cell-extracellular matrix interactions regulate neural differentiation of human embryonic stem cells. BMC Dev Biol. 8:90. DOI: 10.1186/1471-213X-8-90. PMID: 18808690. PMCID: PMC2570688.
Article60. Kothapalli CR, Kamm RD. 2013; 3D matrix microenvironment for targeted differentiation of embryonic stem cells into neural and glial lineages. Biomaterials. 34:5995–6007. DOI: 10.1016/j.biomaterials.2013.04.042. PMID: 23694902.
Article61. Sood D, Cairns DM, Dabbi JM, Ramakrishnan C, Deisseroth K, Black LD 3rd, Santaniello S, Kaplan DL. 2019; Functional maturation of human neural stem cells in a 3D bioengineered brain model enriched with fetal brain-derived matrix. Sci Rep. 9:17874. DOI: 10.1038/s41598-019-54248-1. PMID: 31784595. PMCID: PMC6884597.
Article62. Zhang ZN, Freitas BC, Qian H, Lux J, Acab A, Trujillo CA, Herai RH, Nguyen Huu VA, Wen JH, Joshi-Barr S, Karpiak JV, Engler AJ, Fu XD, Muotri AR, Almutairi A. 2016; Layered hydrogels accelerate iPSC-derived neuronal maturation and reveal migration defects caused by MeCP2 dysfunction. Proc Natl Acad Sci U S A. 113:3185–3190. DOI: 10.1073/pnas.1521255113. PMID: 26944080. PMCID: PMC4812712.
Article63. Bozza A, Coates EE, Incitti T, Ferlin KM, Messina A, Menna E, Bozzi Y, Fisher JP, Casarosa S. 2014; Neural differentiation of pluripotent cells in 3D alginate-based cultures. Biomaterials. 35:4636–4645. DOI: 10.1016/j.biomaterials.2014.02.039. PMID: 24631250.
Article64. Lindborg BA, Brekke JH, Vegoe AL, Ulrich CB, Haider KT, Subramaniam S, Venhuizen SL, Eide CR, Orchard PJ, Chen W, Wang Q, Pelaez F, Scott CM, Kokkoli E, Keirstead SA, Dutton JR, Tolar J, O'Brien TD. 2016; Rapid induction of cerebral organoids from human induced pluripotent stem cells using a chemically defined hydrogel and defined cell culture medium. Stem Cells Transl Med. 5:970–979. DOI: 10.5966/sctm.2015-0305. PMID: 27177577. PMCID: PMC4922855.
Article65. Lam J, Carmichael ST, Lowry WE, Segura T. 2015; Hydrogel design of experiments methodology to optimize hydrogel for iPSC-NPC culture. Adv Healthc Mater. 4:534–539. DOI: 10.1002/adhm.201400410. PMID: 25378176. PMCID: PMC4384641.
Article66. Ranga A, Gobaa S, Okawa Y, Mosiewicz K, Negro A, Lutolf MP. 2014; 3D niche microarrays for systems-level analyses of cell fate. Nat Commun. 5:4324. DOI: 10.1038/ncomms5324. PMID: 25027775. PMCID: PMC4104440.
Article67. Ranga A, Girgin M, Meinhardt A, Eberle D, Caiazzo M, Tanaka EM, Lutolf MP. 2016; Neural tube morphogenesis in synthetic 3D microenvironments. Proc Natl Acad Sci U S A. 113:E6831–E6839. Erratum in: Proc Natl Acad Sci U S A 2017;114:E3163. DOI: 10.1073/pnas.1603529113. PMID: 27742791. PMCID: PMC5098636.
Article68. Qian X, Jacob F, Song MM, Nguyen HN, Song H, Ming GL. 2018; Generation of human brain region-specific organoids using a miniaturized spinning bioreactor. Nat Protoc. 13:565–580. DOI: 10.1038/nprot.2017.152. PMID: 29470464. PMCID: PMC6241211.
Article69. DiStefano T, Chen HY, Panebianco C, Kaya KD, Brooks MJ, Gieser L, Morgan NY, Pohida T, Swaroop A. 2018; Accelerated and improved differentiation of retinal organoids from pluripotent stem cells in rotating-wall vessel bioreactors. Stem Cell Reports. 10:300–313. Erratum in: Stem Cell Reports 2021;16:224. DOI: 10.1016/j.stemcr.2020.12.006. PMID: 33440179. PMCID: PMC7897579.
Article70. Zhong X, Gutierrez C, Xue T, Hampton C, Vergara MN, Cao LH, Peters A, Park TS, Zambidis ET, Meyer JS, Gamm DM, Yau KW, Canto-Soler MV. 2014; Generation of three-dimensional retinal tissue with functional photoreceptors from human iPSCs. Nat Commun. 5:4047. DOI: 10.1038/ncomms5047. PMID: 24915161. PMCID: PMC4370190.
Article71. Brooks MJ, Chen HY, Kelley RA, Mondal AK, Nagashima K, De Val N, Li T, Chaitankar V, Swaroop A. 2019; Improved retinal organoid differentiation by modulating signaling pathways revealed by comparative transcriptome analyses with development in vivo. Stem Cell Reports. 13:891–905. DOI: 10.1016/j.stemcr.2019.09.009. PMID: 31631019. PMCID: PMC6895716.
Article72. Giandomenico SL, Mierau SB, Gibbons GM, Wenger LMD, Masullo L, Sit T, Sutcliffe M, Boulanger J, Tripodi M, Derivery E, Paulsen O, Lakatos A, Lancaster MA. 2019; Cerebral organoids at the air-liquid interface generate diverse nerve tracts with functional output. Nat Neurosci. 22:669–679. DOI: 10.1038/s41593-019-0350-2. PMID: 30886407. PMCID: PMC6436729.
Article73. Szebényi K, Wenger LMD, Sun Y, Dunn AWE, Limegrover CA, Gibbons GM, Conci E, Paulsen O, Mierau SB, Balmus G, Lakatos A. 2021; Human ALS/FTD brain organoid slice cultures display distinct early astrocyte and targetable neuronal pathology. Nat Neurosci. 24:1542–1554. DOI: 10.1038/s41593-021-00923-4. PMID: 34675437. PMCID: PMC8553627.
Article74. Qian X, Su Y, Adam CD, Deutschmann AU, Pather SR, Goldberg EM, Su K, Li S, Lu L, Jacob F, Nguyen PTT, Huh S, Hoke A, Swinford-Jackson SE, Wen Z, Gu X, Pierce RC, Wu H, Briand LA, Chen HI, Wolf JA, Song H, Ming GL. 2020; Sliced human cortical organoids for modeling distinct cortical layer formation. Cell Stem Cell. 26:766–781.e9. DOI: 10.1016/j.stem.2020.02.002. PMID: 32142682. PMCID: PMC7366517.
Article75. Miller JS, Stevens KR, Yang MT, Baker BM, Nguyen DH, Cohen DM, Toro E, Chen AA, Galie PA, Yu X, Chaturvedi R, Bhatia SN, Chen CS. 2012; Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat Mater. 11:768–774. DOI: 10.1038/nmat3357. PMID: 22751181. PMCID: PMC3586565.
Article76. Mirabella T, MacArthur JW, Cheng D, Ozaki CK, Woo YJ, Yang M, Chen CS. 2017; 3D-printed vascular networks direct therapeutic angiogenesis in ischaemia. Nat Biomed Eng. 1:0083. Erratum in: Nat Biomed Eng 2020;4:572. DOI: 10.1038/s41551-020-0552-7. PMID: 32251393. PMCID: PMC5837070.
Article77. Pham MT, Pollock KM, Rose MD, Cary WA, Stewart HR, Zhou P, Nolta JA, Waldau B. 2018; Generation of human vascularized brain organoids. Neuroreport. 29:588–593. DOI: 10.1097/WNR.0000000000001014. PMID: 29570159. PMCID: PMC6476536.
Article78. Shi Y, Sun L, Wang M, Liu J, Zhong S, Li R, Li P, Guo L, Fang A, Chen R, Ge WP, Wu Q, Wang X. 2020; Vascularized human cortical organoids (vOrganoids) model cortical development in vivo. PLoS Biol. 18:e3000705. DOI: 10.1371/journal.pbio.3000705. PMID: 32401820. PMCID: PMC7250475. PMID: 66f6d290c2f84ede886185d58cd7368f.
Article79. Cakir B, Xiang Y, Tanaka Y, Kural MH, Parent M, Kang YJ, Chapeton K, Patterson B, Yuan Y, He CS, Raredon MSB, Dengelegi J, Kim KY, Sun P, Zhong M, Lee S, Patra P, Hyder F, Niklason LE, Lee SH, Yoon YS, Park IH. 2019; Engineering of human brain organoids with a functional vascular-like system. Nat Methods. 16:1169–1175. DOI: 10.1038/s41592-019-0586-5. PMID: 31591580. PMCID: PMC6918722.
Article80. Cho AN, Jin Y, An Y, Kim J, Choi YS, Lee JS, Kim J, Choi WY, Koo DJ, Yu W, Chang GE, Kim DY, Jo SH, Kim J, Kim SY, Kim YG, Kim JY, Choi N, Cheong E, Kim YJ, Je HS, Kang HC, Cho SW. 2021; Microfluidic device with brain extracellular matrix promotes structural and functional maturation of human brain organoids. Nat Commun. 12:4730. DOI: 10.1038/s41467-021-24775-5. PMID: 34354063. PMCID: PMC8342542. PMID: 4baaa6ea520348a5bdc13ee2f2a1412c.
Article81. Wang Y, Wang L, Guo Y, Zhu Y, Qin J. 2018; Engineering stem cell-derived 3D brain organoids in a perfusable organ-on-a-chip system. RSC Adv. 8:1677–1685. DOI: 10.1039/C7RA11714K.
Article82. Berger E, Magliaro C, Paczia N, Monzel AS, Antony P, Linster CL, Bolognin S, Ahluwalia A, Schwamborn JC. 2018; Millifluidic culture improves human midbrain organoid vitality and differentiation. Lab Chip. 18:3172–3183. DOI: 10.1039/C8LC00206A. PMID: 30204191.
Article83. Mansour AA, Gonçalves JT, Bloyd CW, Li H, Fernandes S, Quang D, Johnston S, Parylak SL, Jin X, Gage FH. 2018; An in vivo model of functional and vascularized human brain organoids. Nat Biotechnol. 36:432–441. DOI: 10.1038/nbt.4127. PMID: 29658944. PMCID: PMC6331203.
Article84. Wolf SA, Boddeke HW, Kettenmann H. 2017; Microglia in physiology and disease. Annu Rev Physiol. 79:619–643. DOI: 10.1146/annurev-physiol-022516-034406. PMID: 27959620.
Article85. Casano AM, Peri F. 2015; Microglia: multitasking specialists of the brain. Dev Cell. 32:469–477. DOI: 10.1016/j.devcel.2015.01.018. PMID: 25710533.
Article86. Ormel PR, Vieira de Sá R, van Bodegraven EJ, Karst H, Harschnitz O, Sneeboer MAM, Johansen LE, van Dijk RE, Scheefhals N, Berdenis van Berlekom A, Ribes Martínez E, Kling S, MacGillavry HD, van den Berg LH, Kahn RS, Hol EM, de Witte LD, Pasterkamp RJ. 2018; Microglia innately develop within cerebral organoids. Nat Commun. 9:4167. DOI: 10.1038/s41467-018-06684-2. PMID: 30301888. PMCID: PMC6177485. PMID: 0f43c1e80fff44ada197169a4b75a7dc.
Article87. Xu R, Boreland AJ, Li X, Erickson C, Jin M, Atkins C, Pang ZP, Daniels BP, Jiang P. 2021; Developing human pluripotent stem cell-based cerebral organoids with a controllable microglia ratio for modeling brain development and pathology. Stem Cell Reports. 16:1923–1937. DOI: 10.1016/j.stemcr.2021.06.011. PMID: 34297942. PMCID: PMC8365109.
Article88. Siegenthaler JA, Ashique AM, Zarbalis K, Patterson KP, Hecht JH, Kane MA, Folias AE, Choe Y, May SR, Kume T, Napoli JL, Peterson AS, Pleasure SJ. 2009; Retinoic acid from the meninges regulates cortical neuron generation. Cell. 139:597–609. Erratum in: Cell 2011;146:486. DOI: 10.1016/j.cell.2011.07.011. PMID: 19879845. PMCID: PMC2772834.
Article89. D'Arcangelo G, Miao GG, Chen SC, Soares HD, Morgan JI, Curran T. 1995; A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature. 374:719–723. DOI: 10.1038/374719a0. PMID: 7715726.90. D'Arcangelo G, Nakajima K, Miyata T, Ogawa M, Mikoshiba K, Curran T. 1997; Reelin is a secreted glycoprotein recognized by the CR-50 monoclonal antibody. J Neurosci. 17:23–31. DOI: 10.1523/JNEUROSCI.17-01-00023.1997. PMID: 8987733. PMCID: PMC6793694.91. Del Río JA, Heimrich B, Borrell V, Förster E, Drakew A, Alcántara S, Nakajima K, Miyata T, Ogawa M, Mikoshiba K, Derer P, Frotscher M, Soriano E. 1997; A role for Cajal-Retzius cells and reelin in the development of hippocampal connections. Nature. 385:70–74. DOI: 10.1038/385070a0. PMID: 8985248.
Article92. Jacob F, Pather SR, Huang WK, Zhang F, Wong SZH, Zhou H, Cubitt B, Fan W, Chen CZ, Xu M, Pradhan M, Zhang DY, Zheng W, Bang AG, Song H, Carlos de la Torre J, Ming GL. 2020; Human pluripotent stem cell-derived neural cells and brain organoids reveal SARS-CoV-2 neurotropism predominates in choroid plexus epithelium. Cell Stem Cell. 27:937–950.e9. DOI: 10.1016/j.stem.2020.09.016. PMID: 33010822. PMCID: PMC7505550.
Article93. Pellegrini L, Albecka A, Mallery DL, Kellner MJ, Paul D, Carter AP, James LC, Lancaster MA. 2020; SARS-CoV-2 infects the brain choroid plexus and disrupts the blood-CSF barrier in human brain organoids. Cell Stem Cell. 27:951–961.e5. DOI: 10.1016/j.stem.2020.10.001. PMID: 33113348. PMCID: PMC7553118.
Article94. Faustino Martins JM, Fischer C, Urzi A, Vidal R, Kunz S, Ruffault PL, Kabuss L, Hube I, Gazzerro E, Birchmeier C, Spuler S, Sauer S, Gouti M. 2020; Self-organizing 3D human trunk neuromuscular organoids. Cell Stem Cell. 27:498. Erratum for: Cell Stem Cell 2020;26:172-186.e6. DOI: 10.1016/j.stem.2019.12.007. PMID: 31956040.
Article95. Wagner DE, Klein AM. 2020; Lineage tracing meets single-cell omics: opportunities and challenges. Nat Rev Genet. 21:410–427. DOI: 10.1038/s41576-020-0223-2. PMID: 32235876. PMCID: PMC7307462.
Article96. Keller PJ, Schmidt AD, Wittbrodt J, Stelzer EH. 2008; Reconstruction of zebrafish early embryonic development by scanned light sheet microscopy. Science. 322:1065–1069. DOI: 10.1126/science.1162493. PMID: 18845710.
Article97. McDole K, Guignard L, Amat F, Berger A, Malandain G, Royer LA, Turaga SC, Branson K, Keller PJ. 2018; In toto imaging and reconstruction of post-implantation mouse development at the single-cell level. Cell. 175:859–876.e33. DOI: 10.1016/j.cell.2018.09.031. PMID: 30318151.
Article98. Behjati S, Huch M, van Boxtel R, Karthaus W, Wedge DC, Tamuri AU, Martincorena I, Petljak M, Alexandrov LB, Gundem G, Tarpey PS, Roerink S, Blokker J, Maddison M, Mudie L, Robinson B, Nik-Zainal S, Campbell P, Goldman N, van de Wetering M, Cuppen E, Clevers H, Stratton MR. 2014; Genome sequencing of normal cells reveals developmental lineages and mutational processes. Nature. 513:422–425. DOI: 10.1038/nature13448. PMID: 25043003. PMCID: PMC4227286.
Article99. Lodato MA, Woodworth MB, Lee S, Evrony GD, Mehta BK, Karger A, Lee S, Chittenden TW, D'Gama AM, Cai X, Luquette LJ, Lee E, Park PJ, Walsh CA. 2015; Somatic mutation in single human neurons tracks developmental and transcriptional history. Science. 350:94–98. DOI: 10.1126/science.aab1785. PMID: 26430121. PMCID: PMC4664477.
Article100. Evrony GD, Lee E, Mehta BK, Benjamini Y, Johnson RM, Cai X, Yang L, Haseley P, Lehmann HS, Park PJ, Walsh CA. 2015; Cell lineage analysis in human brain using endogenous retroelements. Neuron. 85:49–59. DOI: 10.1016/j.neuron.2014.12.028. PMID: 25569347. PMCID: PMC4299461.
Article101. Del Dosso A, Urenda JP, Nguyen T, Quadrato G. 2020; Upgrading the physiological relevance of human brain organoids. Neuron. 107:1014–1028. DOI: 10.1016/j.neuron.2020.08.029. PMID: 32970996.
Article102. McKenna A, Gagnon JA. 2019; Recording development with single cell dynamic lineage tracing. Development. 146:dev169730. DOI: 10.1242/dev.169730. PMID: 31249005. PMCID: PMC6602349.
Article103. Sun J, Ramos A, Chapman B, Johnnidis JB, Le L, Ho YJ, Klein A, Hofmann O, Camargo FD. 2014; Clonal dynamics of native haematopoiesis. Nature. 514:322–327. DOI: 10.1038/nature13824. PMID: 25296256. PMCID: PMC4408613.
Article104. Wagner DE, Weinreb C, Collins ZM, Briggs JA, Megason SG, Klein AM. 2018; Single-cell mapping of gene expression landscapes and lineage in the zebrafish embryo. Science. 360:981–987. DOI: 10.1126/science.aar4362. PMID: 29700229. PMCID: PMC6083445.
Article105. Weinreb C, Rodriguez-Fraticelli A, Camargo FD, Klein AM. 2020; Lineage tracing on transcriptional landscapes links state to fate during differentiation. Science. 367:eaaw3381. DOI: 10.1126/science.aaw3381. PMID: 31974159. PMCID: PMC7608074.
Article106. Biddy BA, Kong W, Kamimoto K, Guo C, Waye SE, Sun T, Morris SA. 2018; Single-cell mapping of lineage and identity in direct reprogramming. Nature. 564:219–224. DOI: 10.1038/s41586-018-0744-4. PMID: 30518857. PMCID: PMC6635140.
Article107. Pei W, Feyerabend TB, Rössler J, Wang X, Postrach D, Busch K, Rode I, Klapproth K, Dietlein N, Quedenau C, Chen W, Sauer S, Wolf S, Höfer T, Rodewald HR. 2017; Polylox barcoding reveals haematopoietic stem cell fates realized in vivo. Nature. 548:456–460. DOI: 10.1038/nature23653. PMID: 28813413. PMCID: PMC5905670.
Article108. Frieda KL, Linton JM, Hormoz S, Choi J, Chow KK, Singer ZS, Budde MW, Elowitz MB, Cai L. 2017; Synthetic recording and in situ readout of lineage information in single cells. Nature. 541:107–111. DOI: 10.1038/nature20777. PMID: 27869821. PMCID: PMC6487260.
Article109. McKenna A, Findlay GM, Gagnon JA, Horwitz MS, Schier AF, Shendure J. 2016; Whole-organism lineage tracing by combinatorial and cumulative genome editing. Science. 353:aaf7907. DOI: 10.1126/science.aaf7907. PMID: 27229144. PMCID: PMC4967023.
Article110. Raj B, Wagner DE, McKenna A, Pandey S, Klein AM, Shendure J, Gagnon JA, Schier AF. 2018; Simultaneous single-cell profiling of lineages and cell types in the vertebrate brain. Nat Biotechnol. 36:442–450. DOI: 10.1038/nbt.4103. PMID: 29608178. PMCID: PMC5938111.
Article111. Alemany A, Florescu M, Baron CS, Peterson-Maduro J, van Oudenaarden A. 2018; Whole-organism clone tracing using single-cell sequencing. Nature. 556:108–112. DOI: 10.1038/nature25969. PMID: 29590089.
Article112. Spanjaard B, Hu B, Mitic N, Olivares-Chauvet P, Janjuha S, Ninov N, Junker JP. 2018; Simultaneous lineage tracing and cell-type identification using CRISPR-Cas9-induced genetic scars. Nat Biotechnol. 36:469–473. DOI: 10.1038/nbt.4124. PMID: 29644996. PMCID: PMC5942543.
Article113. Kalhor R, Kalhor K, Mejia L, Leeper K, Graveline A, Mali P, Church GM. 2018; Developmental barcoding of whole mouse via homing CRISPR. Science. 361:eaat9804. DOI: 10.1126/science.aat9804. PMID: 30093604. PMCID: PMC6139672.
Article114. VanHorn S, Morris SA. 2021; Next-generation lineage tracing and fate mapping to interrogate development. Dev Cell. 56:7–21. DOI: 10.1016/j.devcel.2020.10.021. PMID: 33217333.
Article115. Loveless TB, Grotts JH, Schechter MW, Forouzmand E, Carlson CK, Agahi BS, Liang G, Ficht M, Liu B, Xie X, Liu CC. 2021; Lineage tracing and analog recording in mammalian cells by single-site DNA writing. Nat Chem Biol. 17:739–747. DOI: 10.1038/s41589-021-00769-8. PMID: 33753928.
Article116. He Z, Gerber T, Maynard A, Jain A, Petri R, Santel M, Ly K, Sidow L, Sanchís-Calleja F, Riesenberg S, Camp JG, Treutlein B. 2020. Lineage recording reveals dynamics of cerebral organoid regionalization. bioRxiv 162032 [Preprint]. Available from: https://doi.org/10.1101/2020.06.19.162032. cited 2020 Jun 26. DOI: 10.1101/2020.06.19.162032.
Article117. Camp JG, Badsha F, Florio M, Kanton S, Gerber T, Wilsch-Bräuninger M, Lewitus E, Sykes A, Hevers W, Lancaster M, Knoblich JA, Lachmann R, Pääbo S, Huttner WB, Treutlein B. 2015; Human cerebral organoids recapitulate gene expression programs of fetal neocortex development. Proc Natl Acad Sci U S A. 112:15672–15677. DOI: 10.1073/pnas.1520760112. PMID: 26644564. PMCID: PMC4697386.
Article118. Marton RM, Miura Y, Sloan SA, Li Q, Revah O, Levy RJ, Huguenard JR, Pașca SP. 2019; Differentiation and maturation of oligodendrocytes in human three-dimensional neural cultures. Nat Neurosci. 22:484–491. DOI: 10.1038/s41593-018-0316-9. PMID: 30692691. PMCID: PMC6788758.
Article119. Ziffra RS, Kim CN, Wilfert A, Turner TN, Haeussler M, Casella AM, Przytycki PF, Kreimer A, Pollard KS, Ament SA, Eichler EE, Ahituv N, Nowakowski TJ. 2020. Single cell epigenomic atlas of the developing human brain and organoids. bioRxiv 891549 [Preprint]. Available from: https://doi.org/10.1101/2019.12.30.891549. cited 2020 Jan 8. DOI: 10.1101/2019.12.30.891549. PMID: 32477718. PMCID: PMC7252788.
Article120. Sloan SA, Darmanis S, Huber N, Khan TA, Birey F, Caneda C, Reimer R, Quake SR, Barres BA, Paşca SP. 2017; Human astrocyte maturation captured in 3D cerebral cortical spheroids derived from pluripotent stem cells. Neuron. 95:779–790.e6. DOI: 10.1016/j.neuron.2017.07.035. PMID: 28817799. PMCID: PMC5890820.
Article121. Betjes MA, Zheng X, Kok RNU, van Zon JS, Tans SJ. 2021; Cell tracking for organoids: lessons from developmental biology. Front Cell Dev Biol. 9:675013. DOI: 10.3389/fcell.2021.675013. PMID: 34150770. PMCID: PMC8209328. PMID: 498c79092a70496097d2d953a769c13c.
Article122. Lo YH, Karlsson K, Kuo CJ. 2020; Applications of organoids for cancer biology and precision medicine. Nat Cancer. 1:761–773. DOI: 10.1038/s43018-020-0102-y. PMID: 34142093. PMCID: PMC8208643.
Article123. Sugimoto S, Ohta Y, Fujii M, Matano M, Shimokawa M, Nanki K, Date S, Nishikori S, Nakazato Y, Nakamura T, Kanai T, Sato T. 2018; Reconstruction of the human colon epithelium in vivo. Cell Stem Cell. 22:171–176.e5. DOI: 10.1016/j.stem.2017.11.012. PMID: 29290616.
Article124. Bell C, Fang W, Berlinicke C, Kaushik A, Zhang P, Wang T, Kalhor R, Ji H, Zack D. 2020; Single-cell lineage tracing of developing retinal systems. Invest Ophthalmol Vis Sci. 61:4014.125. Zhang M, Torres Z, Owens D, Suter R, Ayad N, Harbour J, Pelaez D. 2020; Lineage tracing in retinal organoids as a platform for studying retinoblastoma. Invest Ophthalmol Vis Sci. 61:3815.126. Poli D, Magliaro C, Ahluwalia A. 2019; Experimental and computational methods for the study of cerebral organoids: a review. Front Neurosci. 13:162. DOI: 10.3389/fnins.2019.00162. PMID: 30890910. PMCID: PMC6411764.
Article127. Chen BC, Legant WR, Wang K, Shao L, Milkie DE, Davidson MW, Janetopoulos C, Wu XS, Hammer JA 3rd, Liu Z, English BP, Mimori-Kiyosue Y, Romero DP, Ritter AT, Lippincott-Schwartz J, Fritz-Laylin L, Mullins RD, Mitchell DM, Bembenek JN, Reymann AC, Böhme R, Grill SW, Wang JT, Seydoux G, Tulu US, Kiehart DP, Betzig E. 2014; Lattice light-sheet microscopy: imaging molecules to embryos at high spatiotemporal resolution. Science. 346:1257998. DOI: 10.1126/science.1257998. PMID: 25342811. PMCID: PMC4336192.
Article128. Schöneberg J, Dambournet D, Liu TL, Forster R, Hockemeyer D, Betzig E, Drubin DG. 2018; 4D cell biology: big data image analytics and lattice light-sheet imaging reveal dynamics of clathrin-mediated endocytosis in stem cell-derived intestinal organoids. Mol Biol Cell. 29:2959–2968. DOI: 10.1091/mbc.E18-06-0375. PMID: 30188768. PMCID: PMC6329908.
Article129. Lavagnino Z, Sancataldo G, d'Amora M, Follert P, De Pietri Tonelli D, Diaspro A, Cella Zanacchi F. 2016; 4D (x-y-z-t) imaging of thick biological samples by means of two-photon inverted selective plane illumination microscopy (2PE-iSPIM). Sci Rep. 6:23923. DOI: 10.1038/srep23923. PMID: 27033347. PMCID: PMC4817031.
Article130. Sakaguchi H, Ozaki Y, Ashida T, Matsubara T, Oishi N, Kihara S, Takahashi J. 2019; Self-organized synchronous calcium transients in a cultured human neural network derived from cerebral organoids. Stem Cell Reports. 13:458–473. DOI: 10.1016/j.stemcr.2019.05.029. PMID: 31257131. PMCID: PMC6739638.
Article131. Miller EW, Lin JY, Frady EP, Steinbach PA, Kristan WB Jr, Tsien RY. 2012; Optically monitoring voltage in neurons by photo-induced electron transfer through molecular wires. Proc Natl Acad Sci U S A. 109:2114–2119. DOI: 10.1073/pnas.1120694109. PMID: 22308458. PMCID: PMC3277584.
Article132. Yan P, Acker CD, Zhou WL, Lee P, Bollensdorff C, Negrean A, Lotti J, Sacconi L, Antic SD, Kohl P, Mansvelder HD, Pavone FS, Loew LM. 2012; Palette of fluorinated voltage-sensitive hemicyanine dyes. Proc Natl Acad Sci U S A. 109:20443–20448. DOI: 10.1073/pnas.1214850109. PMID: 23169660. PMCID: PMC3528613.
Article133. Hochbaum DR, Zhao Y, Farhi SL, Klapoetke N, Werley CA, Kapoor V, Zou P, Kralj JM, Maclaurin D, Smedemark-Margulies N, Saulnier JL, Boulting GL, Straub C, Cho YK, Melkonian M, Wong GK, Harrison DJ, Murthy VN, Sabatini BL, Boyden ES, Campbell RE, Cohen AE. 2014; All-optical electrophysiology in mammalian neurons using engineered microbial rhodopsins. Nat Methods. 11:825–833. DOI: 10.1038/nmeth.3000. PMID: 24952910. PMCID: PMC4117813.
Article134. Puppo F, Sadegh S, Trujillo CA, Thunemann M, Campbell EP, Vandenberghe M, Shan X, Akkouh IA, Miller EW, Bloodgood BL, Silva GA, Dale AM, Einevoll GT, Djurovic S, Andreassen OA, Muotri AR, Devor A. 2021; All-optical electrophysiology in hiPSC-derived neurons with synthetic voltage sensors. Front Cell Neurosci. 15:671549. DOI: 10.3389/fncel.2021.671549. PMID: 34122014. PMCID: PMC8193062. PMID: 9cc7f85830ed4333932e3c468dab069e.
Article135. Trujillo CA, Gao R, Negraes PD, Gu J, Buchanan J, Preissl S, Wang A, Wu W, Haddad GG, Chaim IA, Domissy A, Vandenberghe M, Devor A, Yeo GW, Voytek B, Muotri AR. 2019; Complex oscillatory waves emerging from cortical organoids model early human brain network development. Cell Stem Cell. 25:558–569.e7. DOI: 10.1016/j.stem.2019.08.002. PMID: 31474560. PMCID: PMC6778040.
Article136. Fair SR, Julian D, Hartlaub AM, Pusuluri ST, Malik G, Summerfied TL, Zhao G, Hester AB, Ackerman WE 4th, Hollingsworth EW, Ali M, McElroy CA, Buhimschi IA, Imitola J, Maitre NL, Bedrosian TA, Hester ME. 2020; Electrophysiological maturation of cerebral organoids correlates with dynamic morphological and cellular development. Stem Cell Reports. 15:855–868. DOI: 10.1016/j.stemcr.2020.08.017. PMID: 32976764. PMCID: PMC7562943.
Article137. Soscia DA, Lam D, Tooker AC, Enright HA, Triplett M, Karande P, Peters SKG, Sales AP, Wheeler EK, Fischer NO. 2020; A flexible 3-dimensional microelectrode array for in vitro brain models. Lab Chip. 20:901–911. DOI: 10.1039/C9LC01148J. PMID: 31976505.
Article138. Didier CM, Kundu A, DeRoo D, Rajaraman S. 2020; Development of in vitro 2D and 3D microelectrode arrays and their role in advancing biomedical research. J Micromech Microeng. 30:103001. DOI: 10.1088/1361-6439/ab8e91.
Article139. Yuan X, Schröter M, Obien MEJ, Fiscella M, Gong W, Kikuchi T, Odawara A, Noji S, Suzuki I, Takahashi J, Hierlemann A, Frey U. 2020; Versatile live-cell activity analysis platform for characterization of neuronal dynamics at single-cell and network level. Nat Commun. 11:4854. DOI: 10.1038/s41467-020-18620-4. PMID: 32978383. PMCID: PMC7519655. PMID: a0577c2ed2304ba1ab8ed0c9e23fe0c6.
Article140. Georgiou M, Chichagova V, Hilgen G, Dorgau B, Sernagor E, Armstrong L, Lako M. 2020; Room temperature shipment does not affect the biological activity of pluripotent stem cell-derived retinal organoids. PLoS One. 15:e0233860. DOI: 10.1371/journal.pone.0233860. PMID: 32479513. PMCID: PMC7263587. PMID: 94c41d09b84e44518d9a9abf9646d13b.
Article141. Mellough CB, Collin J, Queen R, Hilgen G, Dorgau B, Zerti D, Felemban M, White K, Sernagor E, Lako M. 2019; Systematic comparison of retinal organoid differentiation from human pluripotent stem cells reveals stage specific, cell line, and methodological differences. Stem Cells Transl Med. 8:694–706. DOI: 10.1002/sctm.18-0267. PMID: 30916455. PMCID: PMC6591558.
Article142. Shin H, Jeong S, Lee JH, Sun W, Choi N, Cho IJ. 2021; 3D high-density microelectrode array with optical stimulation and drug delivery for investigating neural circuit dynamics. Nat Commun. 12:492. DOI: 10.1038/s41467-020-20763-3. PMID: 33479237. PMCID: PMC7820464. PMID: b7878afc0e8744b9ab07f36f00339340.
Article143. Shim C, Jo Y, Cha HK, Kim MK, Kim H, Kook G, Kim K, Son GH, Lee HJ. 2020. Highly stretchable microelectrode array for free-form 3D neuronal tissue. Paper presented at: 2020 IEEE 33rd International Conference on Micro Electro Mechanical Systems (MEMS). 2020 Jan 18-22; Vancouver, Canada. DOI: 10.1109/MEMS46641.2020.9056250. PMID: 34567573. PMCID: PMC8460092.
Article144. Park Y, Franz CK, Ryu H, Luan H, Cotton KY, Kim JU, Chung TS, Zhao S, Vazquez-Guardado A, Yang DS, Li K, Avila R, Phillips JK, Quezada MJ, Jang H, Kwak SS, Won SM, Kwon K, Jeong H, Bandodkar AJ, Han M, Zhao H, Osher GR, Wang H, Lee K, Zhang Y, Huang Y, Finan JD, Rogers JA. 2021; Three-dimensional, multifunctional neural interfaces for cortical spheroids and engineered assembloids. Sci Adv. 7:eabf9153. DOI: 10.1126/sciadv.abf9153. PMID: 33731359. PMCID: PMC7968849.
Article145. Adly N, Weidlich S, Seyock S, Brings F, Yakushenko A, Offenhäusser A, Wolfrum B. 2018; Printed microelectrode arrays on soft materials: from PDMS to hydrogels. npj Flex Electron. 2:15. DOI: 10.1038/s41528-018-0027-z. PMID: ca02f5e1127145a9ad370c9dc716b7d4.
Article146. Ryynänen T, Pelkonen A, Grigoras K, Ylivaara OME, Hyvärinen T, Ahopelto J, Prunnila M, Narkilahti S, Lekkala J. 2019; Microelectrode array with transparent ALD TiN electrodes. Front Neurosci. 13:226. DOI: 10.3389/fnins.2019.00226. PMID: 30967754. PMCID: PMC6438859.
Article147. Atmaramani R, Chakraborty B, Rihani RT, Usoro J, Hammack A, Abbott J, Nnoromele P, Black BJ, Pancrazio JJ, Cogan SF. 2020; Ruthenium oxide based microelectrode arrays for in vitro and in vivo neural recording and stimulation. Acta Biomater. 101:565–574. DOI: 10.1016/j.actbio.2019.10.040. PMID: 31678740. PMCID: PMC7197741.
Article148. Li Q, Nan K, Le Floch P, Lin Z, Sheng H, Blum TS, Liu J. 2019; Cyborg organoids: implantation of nanoelectronics via organogenesis for tissue-wide electrophysiology. Nano Lett. 19:5781–5789. DOI: 10.1021/acs.nanolett.9b02512. PMID: 31347851.
Article149. Le Floch P, Li Q, Liu R, Tasnim K, Zhao S, Lin Z, Jiang H, Liu J. 2021. A method for three-dimensional single-cell chronic electrophysiology from developing brain organoids. bioRxiv 449502 [Preprint]. Available from: https://doi.org/10.1101/2021.06.22.449502. cited 2021 Jun 21. DOI: 10.1101/2021.06.22.449502.
Article150. Li H, Wang J, Fang Y. 2020; Bioinspired flexible electronics for seamless neural interfacing and chronic recording. Nanoscale Adv. 2:3095–3102. DOI: 10.1039/D0NA00323A.
Article151. Tian B, Liu J, Dvir T, Jin L, Tsui JH, Qing Q, Suo Z, Langer R, Kohane DS, Lieber CM. 2012; Macroporous nanowire nanoelectronic scaffolds for synthetic tissues. Nat Mater. 11:986–994. DOI: 10.1038/nmat3404. PMID: 22922448. PMCID: PMC3623694.
Article152. Yang X, Zhou T, Zwang TJ, Hong G, Zhao Y, Viveros RD, Fu TM, Gao T, Lieber CM. 2019; Bioinspired neuron-like electronics. Nat Mater. 18:510–517. DOI: 10.1038/s41563-019-0292-9. PMID: 30804509. PMCID: PMC6474791.
Article153. Miccoli B, Lopez CM, Goikoetxea E, Putzeys J, Sekeri M, Krylychkina O, Chang SW, Firrincieli A, Andrei A, Reumers V, Braeken D. 2019; High-density electrical recording and impedance imaging with a multi-modal CMOS multi-electrode array chip. Front Neurosci. 13:641. DOI: 10.3389/fnins.2019.00641. PMID: 31293372. PMCID: PMC6603149.
Article154. Li Q, Lin Z, Liu R, Tang X, Huang J, He Y, Zhou H, Sheng H, Shi H, Wang X, Liu J. 2021. In situ electro-sequencing in three-dimensional tissues. bioRxiv 440941 [Preprint]. Available from: https://doi.org/10.1101/2021.04.22.440941. cited 2021 Apr 23. DOI: 10.1101/2021.04.22.440941.
Article155. Zou L, Tian H, Guan S, Ding J, Gao L, Wang J, Fang Y. 2021; Self-assembled multifunctional neural probes for precise integration of optogenetics and electrophysiology. Nat Commun. 12:5871. DOI: 10.1038/s41467-021-26168-0. PMID: 34620851. PMCID: PMC8497603. PMID: 7d4c4e68cf9549b585b722c3f10c30e0.
Article156. Shiri Z, Simorgh S, Naderi S, Baharvand H. 2019; Optogenetics in the era of cerebral organoids. Trends Biotechnol. 37:1282–1294. DOI: 10.1016/j.tibtech.2019.05.009. PMID: 31227305.
Article157. Berglund K, Tung JK, Higashikubo B, Gross RE, Moore CI, Hochgeschwender U. 2016; Combined optogenetic and chemogenetic control of neurons. Methods Mol Biol. 1408:207–225. DOI: 10.1007/978-1-4939-3512-3_14. PMID: 26965125. PMCID: PMC5149414.
Article158. Ngo HB, Melo MR, Layfield S, Connelly AA, Bassi JK, Xie L, Menuet C, McDougall SJ, Bathgate RAD, Allen AM. 2020; A chemogenetic tool that enables functional neural circuit analysis. Cell Rep. 32:108139. DOI: 10.1016/j.celrep.2020.108139. PMID: 32937120.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Region Specific Brain Organoids to Study Neurodevelopmental Disorders
- Engineering Human Brain Organoids: From Basic Research to Tissue Regeneration
- A Simple Method for Generating Cerebral Organoids from Human Pluripotent Stem Cells
- Current Challenges Associated with the Use of Human Induced Pluripotent Stem Cell-Derived Organoids in Regenerative Medicine
- Generation of Cortical Brain Organoid with Vascularization by Assembling with Vascular Spheroid