Nutr Res Pract.
2022 Feb;16(1):14-32. 10.4162/nrp.2022.16.1.14.
Gynostemma pentaphyllum extract and Gypenoside L enhance skeletal muscle differentiation and mitochondrial metabolism by activating the PGC-1α pathway in C2C12 myotubes
- Affiliations
-
- 1Technology Development Center, BTC Corporation, Ansan 15588, Korea
- 2Regional Strategic Industry Innovation Center, Hallym University, Chuncheon 24252, Korea
- KMID: 2525509
- DOI: http://doi.org/10.4162/nrp.2022.16.1.14
Abstract
- BACKGROUND/OBJECTIVES
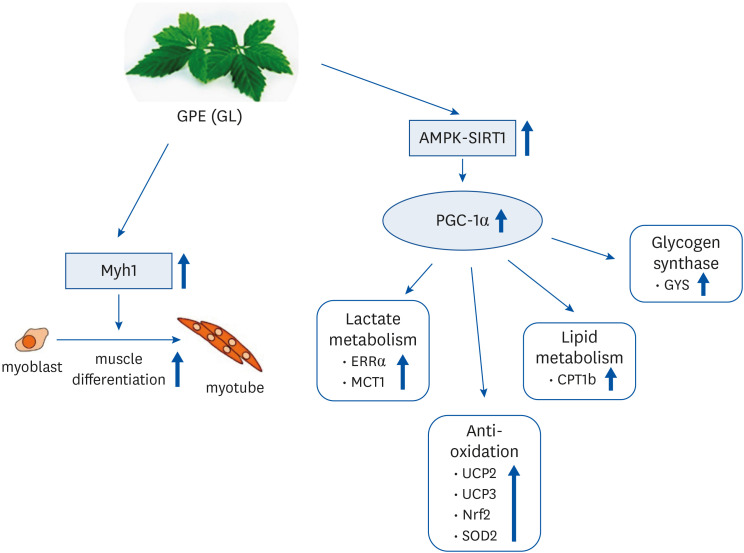
Peroxisome proliferator-activated receptor-gamma co-activator-1α (PGC-1α) has a central role in regulating muscle differentiation and mitochondrial metabolism. PGC-1α stimulates muscle growth and muscle fiber remodeling, concomitantly regulating lactate and lipid metabolism and promoting oxidative metabolism. Gynostemma pentaphyllum (Thumb.) has been widely employed as a traditional herbal medicine and possesses antioxidant, anti-obesity, anti-inflammatory, hypolipemic, hypoglycemic, and anticancer properties. We investigated whether G. pentaphyllum extract (GPE) and its active compound, gypenoside L (GL), affect muscle differentiation and mitochondrial metabolism via activation of the PGC-1α pathway in murine C2C12 myoblast cells.
MATERIALS/METHODS
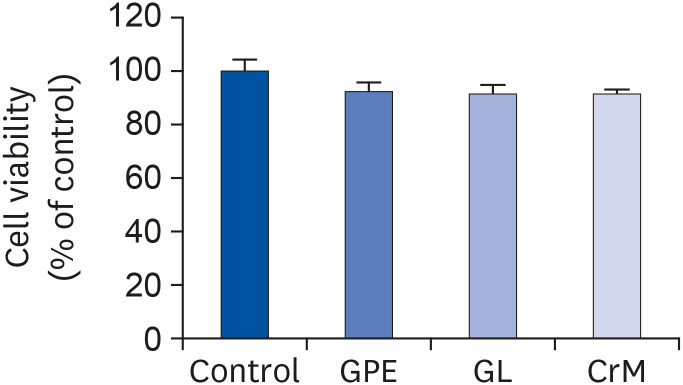
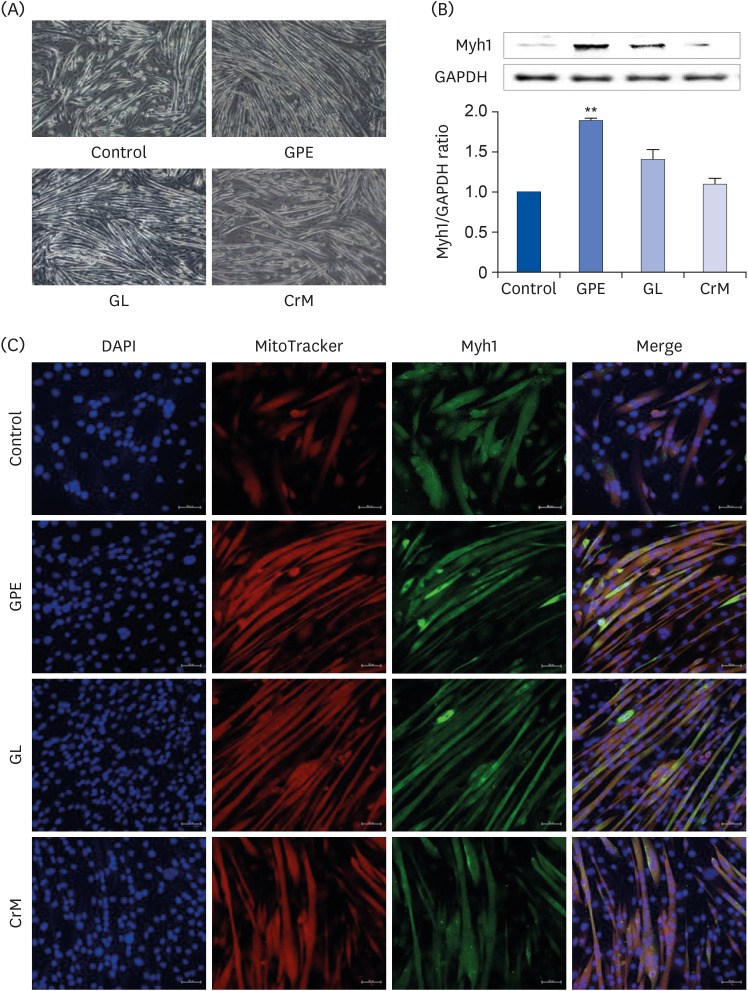
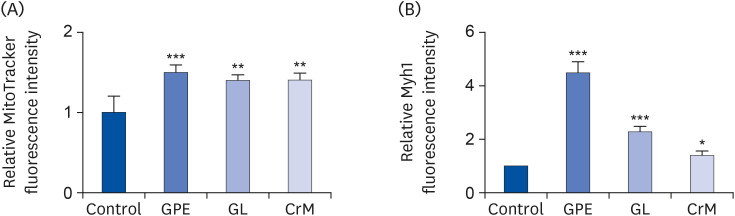
C2C12 cells were treated with GPE and GL, and quantitative reverse transcription polymerase chain reaction and western blot were used to analyze the mRNA and protein expression levels. Myh1 was determined using immunocytochemistry. Mitochondrial reactive oxygen species generation was measured using the 2′7′-dichlorofluorescein diacetate assay.
RESULTS
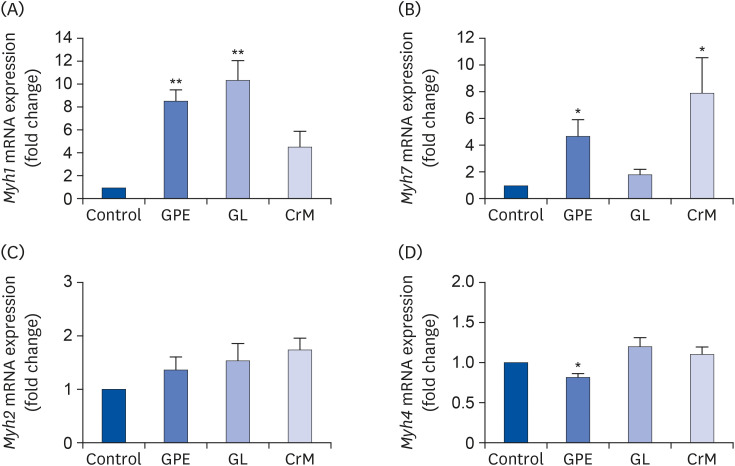
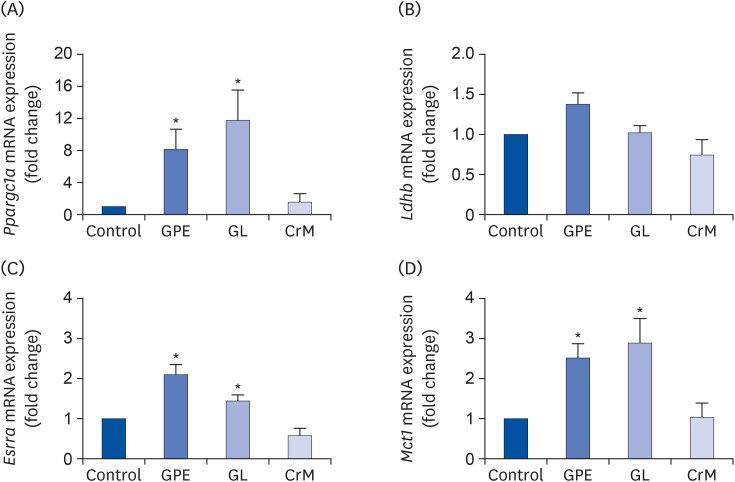
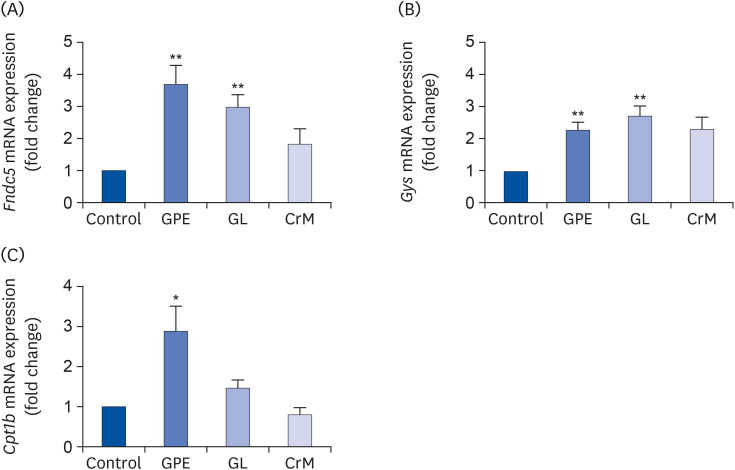
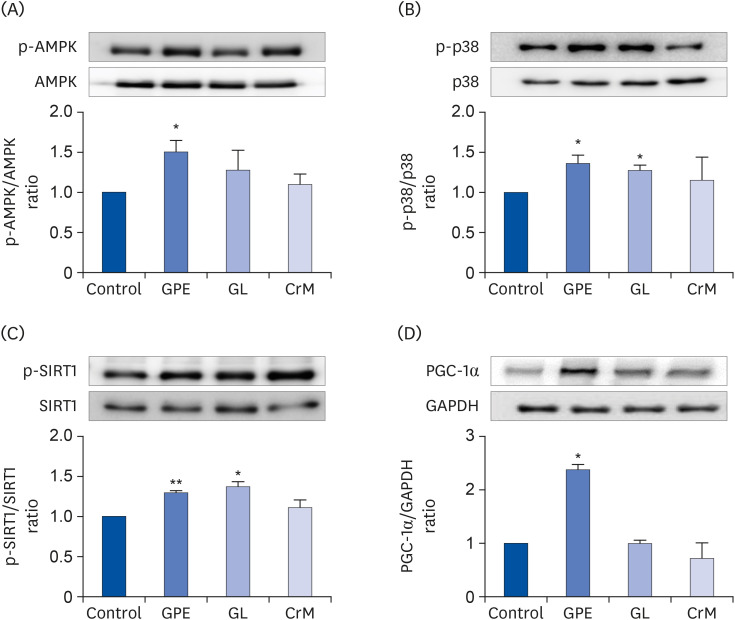
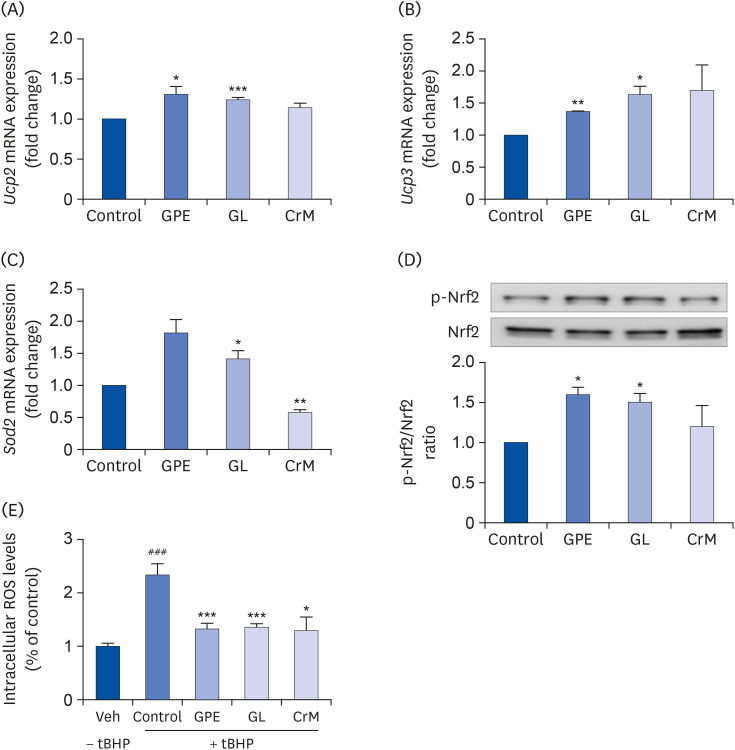
GPE and GL promoted the differentiation of myoblasts into myotubes and elevated mRNA and protein expression levels of Myh1 (type IIx). GPE and GL also significantly increased the mRNA expression levels of the PGC-1α gene (Ppargc1a), lactate metabolismregulatory genes (Esrra and Mct1), adipocyte-browning gene fibronectin type III domaincontaining 5 gene (Fndc5), glycogen synthase gene (Gys), and lipid metabolism gene carnitine palmitoyltransferase 1b gene (Cpt1b). Moreover, GPE and GL induced the phosphorylation of AMP-activated protein kinase, p38, sirtuin1, and deacetylated PGC-1α. We also observed that treatment with GPE and GL significantly stimulated the expression of genes associated with the anti-oxidative stress response, such as Ucp2, Ucp3, Nrf2, and Sod2<>/i>.
CONCLUSIONS
The results indicated that GPE and GL enhance exercise performance by promoting myotube differentiation and mitochondrial metabolism through the upregulation of PGC-1α in C2C12 skeletal muscle.
Keyword
Figure
Reference
-
1. Papa EV, Dong X, Hassan M. Skeletal muscle function deficits in the elderly: current perspectives on resistance training. J Nat Sci. 2017; 3:e272. PMID: 28191501.2. Egan B, Zierath JR. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 2013; 17:162–184. PMID: 23395166.
Article3. Zurlo F, Larson K, Bogardus C, Ravussin E. Skeletal muscle metabolism is a major determinant of resting energy expenditure. J Clin Invest. 1990; 86:1423–1427. PMID: 2243122.
Article4. Margolis LM, Pasiakos SM. Optimizing intramuscular adaptations to aerobic exercise: effects of carbohydrate restriction and protein supplementation on mitochondrial biogenesis. Adv Nutr. 2013; 4:657–664. PMID: 24228194.
Article5. Kang C, Chung E, Diffee G, Ji LL. Exercise training attenuates aging-associated mitochondrial dysfunction in rat skeletal muscle: role of PGC-1α. Exp Gerontol. 2013; 48:1343–1350. PMID: 23994518.
Article6. Ashmore CR, Tompkins G, Doerr L. Postnatal development of muscle fiber types in domestic animals. J Anim Sci. 1972; 34:37–41. PMID: 4258174.
Article7. Zhang L, Zhou Y, Wu W, Hou L, Chen H, Zuo B, Xiong Y, Yang J. Skeletal muscle-specific overexpression of PGC-1α induces fiber-type conversion through enhanced mitochondrial respiration and fatty acid oxidation in mice and pigs. Int J Biol Sci. 2017; 13:1152–1162. PMID: 29104506.
Article8. Ferraro E, Giammarioli AM, Chiandotto S, Spoletini I, Rosano G. Exercise-induced skeletal muscle remodeling and metabolic adaptation: redox signaling and role of autophagy. Antioxid Redox Signal. 2014; 21:154–176. PMID: 24450966.
Article9. Santos-Zas I, Cid-Díaz T, González-Sánchez J, Gurriarán-Rodriguez U, Seoane-Mosteiro C, Porteiro B, Nogueiras R, Casabiell X, Relova JL, Gallego R, et al. Obestatin controls skeletal muscle fiber-type determination. Sci Rep. 2017; 7:2137. PMID: 28522824.
Article10. Bourdeau Julien I, Sephton CF, Dutchak PA. Metabolic networks influencing skeletal muscle fiber composition. Front Cell Dev Biol. 2018; 6:125. PMID: 30324104.
Article11. Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999; 98:115–124. PMID: 10412986.
Article12. Tadaishi M, Miura S, Kai Y, Kano Y, Oishi Y, Ezaki O. Skeletal muscle-specific expression of PGC-1α-b, an exercise-responsive isoform, increases exercise capacity and peak oxygen uptake. PLoS One. 2011; 6:e28290. PMID: 22174785.
Article13. Kelly DP, Scarpulla RC. Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev. 2004; 18:357–368. PMID: 15004004.
Article14. Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005; 1:361–370. PMID: 16054085.
Article15. Puigserver P, Spiegelman BM. Peroxisome proliferator-activated receptor-γ coactivator 1 α (PGC-1 α): transcriptional coactivator and metabolic regulator. Endocr Rev. 2003; 24:78–90. PMID: 12588810.16. McConell GK, Ng GP, Phillips M, Ruan Z, Macaulay SL, Wadley GD. Central role of nitric oxide synthase in AICAR and caffeine-induced mitochondrial biogenesis in L6 myocytes. J Appl Physiol (1985). 2010; 108:589–595. PMID: 20044477.
Article17. da Silva W, Machado AS, Souza MA, Mello-Carpes PB, Carpes FP. Effect of green tea extract supplementation on exercise-induced delayed onset muscle soreness and muscular damage. Physiol Behav. 2018; 194:77–82. PMID: 29746891.
Article18. Liu Y, Liu C. Antifatigue and increasing exercise performance of Actinidia arguta crude alkaloids in mice. J Food Drug Anal. 2016; 24:738–745. PMID: 28911611.19. Lee BR, Lee JH, An HJ. Effects of Taraxacum officinale on fatigue and immunological parameters in mice. Molecules. 2012; 17:13253–13265. PMID: 23135630.20. la Cour B, Mølgaard P, Yi Z. Traditional Chinese medicine in treatment of hyperlipidaemia. J Ethnopharmacol. 1995; 46:125–129. PMID: 7650951.
Article21. Gou SH, Huang HF, Chen XY, Liu J, He M, Ma YY, Zhao XN, Zhang Y, Ni JM. Lipid-lowering, hepatoprotective, and atheroprotective effects of the mixture Hong-Qu and gypenosides in hyperlipidemia with NAFLD rats. J Chin Med Assoc. 2016; 79:111–121. PMID: 26842974.
Article22. Wang M, Wang F, Wang Y, Ma X, Zhao M, Zhao C. Metabonomics study of the therapeutic mechanism of Gynostemma pentaphyllum and atorvastatin for hyperlipidemia in rats. PLoS One. 2013; 8:e78731. PMID: 24223845.23. Müller C, Gardemann A, Keilhoff G, Peter D, Wiswedel I, Schild L. Prevention of free fatty acid-induced lipid accumulation, oxidative stress, and cell death in primary hepatocyte cultures by a Gynostemma pentaphyllum extract. Phytomedicine. 2012; 19:395–401. PMID: 22381945.24. Quan Y, Qian MZ. Effect and mechanism of gypenoside on the inflammatory molecular expression in high-fat induced atherosclerosis rats. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2010; 30:403–406. PMID: 20669679.25. Cai H, Liang Q, Ge G. Gypenoside attenuates β amyloid-induced inflammation in N9 microglial cells via SOCS1 signaling. Neural Plast. 2016; 2016:6362707. PMID: 27213058.26. Liou CJ, Huang WC, Kuo ML, Yang RC, Shen JJ. Long-term oral administration of Gynostemma pentaphyllum extract attenuates airway inflammation and Th2 cell activities in ovalbumin-sensitized mice. Food Chem Toxicol. 2010; 48:2592–2598. PMID: 20558230.27. Willems ME, Myers SD, Gault ML, Cook MD. Beneficial physiological effects with blackcurrant intake in endurance athletes. Int J Sport Nutr Exerc Metab. 2015; 25:367–374. PMID: 25811286.
Article28. Wang J, Yang JL, Zhou PP, Meng XH, Shi YP. Further new gypenosides from Jiaogulan (Gynostemma pentaphyllum). J Agric Food Chem. 2017; 65:5926–5934. PMID: 28662582.29. Lin CC, Huang PC, Lin JM. Antioxidant and hepatoprotective effects of Anoectochilus formosanus and Gynostemma pentaphyllum . Am J Chin Med. 2000; 28:87–96. PMID: 10794120.30. Liu J, Zhang L, Ren Y, Gao Y, Kang L, Qiao Q. Anticancer and immunoregulatory activity of Gynostemma pentaphyllum polysaccharides in H22 tumor-bearing mice. Int J Biol Macromol. 2014; 69:1–4. PMID: 24832985.31. Gao D, Zhao M, Qi X, Liu Y, Li N, Liu Z, Bian Y. Hypoglycemic effect of Gynostemma pentaphyllum saponins by enhancing the Nrf2 signaling pathway in STZ-inducing diabetic rats. Arch Pharm Res. 2016; 39:221–230. PMID: 25066072.32. Ding YJ, Tang KJ, Li FL, Hu QL. Effects of gypenosides from Gynostemma pentaphyllum supplementation on exercise-induced fatigue in mice. Afr J Agric Res. 2010; 5:707–711.33. Wang H, Li C, Wu X, Lou X. Effects of Gynostemma pentaphyllum (Thunb.) Makino polysaccharides supplementation on exercise tolerance and oxidative stress induced by exhaustive exercise in rats. Afr J Agric Res. 2012; 7:2632–2638.34. Kim YH, Kim SM, Lee JK, Jo SK, Kim HJ, Cha KM, Lim CY, Moon JM, Kim TY, Kim EJ. Efficacy of Gynostemma pentaphyllum extract in anti-obesity therapy. Rec Nat Prod. 2020; 14:116–128.35. Yoon J, Ham H, Sung J, Kim Y, Choi Y, Lee JS, Jeong HS, Lee J, Kim D. Black rice extract protected HepG2 cells from oxidative stress-induced cell death via ERK1/2 and Akt activation. Nutr Res Pract. 2014; 8:125–131. PMID: 24741394.
Article36. Summermatter S, Santos G, Pérez-Schindler J, Handschin C. Skeletal muscle PGC-1α controls whole-body lactate homeostasis through estrogen-related receptor α-dependent activation of LDH B and repression of LDH A. Proc Natl Acad Sci U S A. 2013; 110:8738–8743. PMID: 23650363.
Article37. Sozen B, Ozturk S, Yaba A, Demir N. The p38 MAPK signalling pathway is required for glucose metabolism, lineage specification and embryo survival during mouse preimplantation development. Mech Dev. 2015; 138:375–398. PMID: 26025760.
Article38. Wright DC, Geiger PC, Han DH, Jones TE, Holloszy JO. Calcium induces increases in peroxisome proliferator-activated receptor γ coactivator-1α and mitochondrial biogenesis by a pathway leading to p38 mitogen-activated protein kinase activation. J Biol Chem. 2007; 282:18793–18799. PMID: 17488713.
Article39. Gerhart-Hines Z, Rodgers JT, Bare O, Lerin C, Kim SH, Mostoslavsky R, Alt FW, Wu Z, Puigserver P. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1α. EMBO J. 2007; 26:1913–1923. PMID: 17347648.
Article40. Rodgers JT, Lerin C, Gerhart-Hines Z, Puigserver P. Metabolic adaptations through the PGC-1 α and SIRT1 pathways. FEBS Lett. 2008; 582:46–53. PMID: 18036349.41. Ishii T, Itoh K, Takahashi S, Sato H, Yanagawa T, Katoh Y, Bannai S, Yamamoto M. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J Biol Chem. 2000; 275:16023–16029. PMID: 10821856.
Article42. Pi J, Bai Y, Reece JM, Williams J, Liu D, Freeman ML, Fahl WE, Shugar D, Liu J, Qu W, et al. Molecular mechanism of human Nrf2 activation and degradation: role of sequential phosphorylation by protein kinase CK2. Free Radic Biol Med. 2007; 42:1797–1806. PMID: 17512459.
Article43. Apopa PL, He X, Ma Q. Phosphorylation of Nrf2 in the transcription activation domain by casein kinase 2 (CK2) is critical for the nuclear translocation and transcription activation function of Nrf2 in IMR-32 neuroblastoma cells. J Biochem Mol Toxicol. 2008; 22:63–76. PMID: 18273910.
Article44. Norberg A, Hoa NK, Liepinsh E, Van Phan D, Thuan ND, Jörnvall H, Sillard R, Ostenson CG. A novel insulin-releasing substance, phanoside, from the plant Gynostemma pentaphyllum . J Biol Chem. 2004; 279:41361–41367. PMID: 15220351.45. Zhang GL, Deng JP, Wang BH, Zhao ZW, Li J, Gao L, Liu BL, Xong JR, Guo XD, Yan ZQ, et al. Gypenosides improve cognitive impairment induced by chronic cerebral hypoperfusion in rats by suppressing oxidative stress and astrocytic activation. Behav Pharmacol. 2011; 22:633–644. PMID: 21897202.
Article46. Yeo J, Kang YJ, Jeon SM, Jung UJ, Lee MK, Song H, Choi MS. Potential hypoglycemic effect of an ethanol extract of Gynostemma pentaphyllum in C57BL/KsJ-db/db mice. J Med Food. 2008; 11:709–716. PMID: 19053864.47. Lu KW, Ma YS, Yu FS, Huang YP, Chu YL, Wu RS, Liao CL, Chueh FS, Chung JG. Gypenosides induce cell death and alter gene expression in human oral cancer HSC-3 cells. Exp Ther Med. 2017; 14:2469–2476. PMID: 28962182.
Article48. Ahmed I, Leach DN, Wohlmuth H, De Voss JJ, Blanchfield JT. Caco-2 cell permeability of flavonoids and saponins from Gynostemma pentaphyllum: the immotal herb. ACS Omega. 2020; 5:21561–21569. PMID: 32905390.49. Zhang L, Gong H, Sun Q, Zhao R, Jia Y. Spermidine-activated satellite cells are associated with hypoacetylation in ACVR2B and Smad3 binding to myogenic genes in mice. J Agric Food Chem. 2018; 66:540–550. PMID: 29224337.
Article50. Ambrosio F, Kadi F, Lexell J, Fitzgerald GK, Boninger ML, Huard J. The effect of muscle loading on skeletal muscle regenerative potential: an update of current research findings relating to aging and neuromuscular pathology. Am J Phys Med Rehabil. 2009; 88:145–155. PMID: 19169178.51. Motohashi N, Asakura A. Molecular regulation of muscle satellite cell self-renewal. J Stem Cell Res Ther. 2012; (Suppl 11):e002. PMID: 24524010.
Article52. Takeda K, Machida M, Kohara A, Omi N, Takemasa T. Effects of citrulline supplementation on fatigue and exercise performance in mice. J Nutr Sci Vitaminol (Tokyo). 2011; 57:246–250. PMID: 21908948.
Article53. Schreiber SN, Emter R, Hock MB, Knutti D, Cardenas J, Podvinec M, Oakeley EJ, Kralli A. The estrogen-related receptor α (ERRα) functions in PPARγ coactivator 1α (PGC-1α)-induced mitochondrial biogenesis. Proc Natl Acad Sci U S A. 2004; 101:6472–6477. PMID: 15087503.
Article54. Kang C, Li Ji L. Role of PGC-1α signaling in skeletal muscle health and disease. Ann N Y Acad Sci. 2012; 1271:110–117. PMID: 23050972.
Article55. Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Boström EA, Choi JH, Long JZ, et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012; 481:463–468. PMID: 22237023.
Article56. Hosseini Farahabadi SS, Ghaedi K, Ghazvini Zadegan F, Karbalaie K, Rabiee F, Nematollahi M, Baharvand H, Nasr-Esfahani MH. ERK1/2 is a key regulator of Fndc5 and PGC1α expression during neural differentiation of mESCs. Neuroscience. 2015; 297:252–261. PMID: 25869623.
Article57. Chen SQ, Ding LN, Zeng NX, Liu HM, Zheng SH, Xu JW, Li RM. Icariin induces irisin/FNDC5 expression in C2C12 cells via the AMPK pathway. Biomed Pharmacother. 2019; 115:108930. PMID: 31055234.
Article58. Liu J, Li Y, Yang P, Wan J, Chang Q, Wang TTY, Lu W, Zhang Y, Wang Q, Yu LL. Gypenosides reduced the risk of overweight and insulin resistance in C57BL/6J mice through modulating adipose thermogenesis and gut microbiota. J Agric Food Chem. 2017; 65:9237–9246. PMID: 28975783.
Article59. Hunter RW, Treebak JT, Wojtaszewski JF, Sakamoto K. Molecular mechanism by which AMP-activated protein kinase activation promotes glycogen accumulation in muscle. Diabetes. 2011; 60:766–774. PMID: 21282366.
Article60. Janzen NR, Whitfield J, Hoffman NJ. Interactive roles for AMPK and glycogen from cellular energy sensing to exercise metabolism. Int J Mol Sci. 2018; 19:3344.
Article61. Ha J, Guan KL, Kim J. AMPK and autophagy in glucose/glycogen metabolism. Mol Aspects Med. 2015; 46:46–62. PMID: 26297963.
Article62. Hingst JR, Bruhn L, Hansen MB, Rosschou MF, Birk JB, Fentz J, Foretz M, Viollet B, Sakamoto K, Færgeman NJ, et al. Exercise-induced molecular mechanisms promoting glycogen supercompensation in human skeletal muscle. Mol Metab. 2018; 16:24–34. PMID: 30093357.
Article63. Ehrenborg E, Krook A. Regulation of skeletal muscle physiology and metabolism by peroxisome proliferator-activated receptor delta. Pharmacol Rev. 2009; 61:373–393. PMID: 19805479.64. Scarpulla RC. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol Rev. 2008; 88:611–638. PMID: 18391175.
Article65. Hayes JD, Dinkova-Kostova AT. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem Sci. 2014; 39:199–218. PMID: 24647116.
Article66. Demine S, Renard P, Arnould T. Mitochondrial uncoupling: a key controller of biological processes in physiology and diseases. Cells. 2019; 8:795.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Gynostemma pentaphyllum extract and its active component gypenoside L improve the exercise performance of treadmill-trained mice
- The effects of blackcurrant extract on TNF-α-induced myotube atrophy
- Resveratrol promotes mitochondrial energy metabolism in exerciseinduced fatigued rats
- Secretion of adenylate kinase 1 is required for extracellular ATP synthesis in C2C12 myotubes
- The effects of Allomyrina dichotoma larval extract on palmitate-induced insulin resistance in skeletal muscle cells