Nutr Res Pract.
2022 Jun;16(3):298-313. 10.4162/nrp.2022.16.3.298.
Gynostemma pentaphyllum extract and its active component gypenoside L improve the exercise performance of treadmill-trained mice
- Affiliations
-
- 1Technology Development Center, BTC Corporation, Ansan 15588, Korea
- 2Regional Strategic Industry Innovation Center, Hallym University, Chuncheon 24252, Korea
- KMID: 2530392
- DOI: http://doi.org/10.4162/nrp.2022.16.3.298
Abstract
- BACKGROUND/OBJECTIVES
The effectiveness of natural compounds in improving athletic ability has attracted attention in both sports and research. Gynostemma pentaphyllum (Thunb.) leaves are used to make traditional herbal medicines in Asia. The active components of G.pentaphyllum, dammarane saponins, or gypenosides, possess a range of biological activities. On the other hand, the anti-fatigue effects from G. pentaphyllum extract (GPE) and its effective compound, gypenoside L (GL), remain to be determined.
MATERIALS/METHODS
This study examined the effects of GPE on fatigue and exercise performance in ICR mice. GPE was administered orally to mice for 6 weeks, with or without treadmill training. The biochemical analysis in serum, glycogen content, mRNA, and protein expressions of the liver and muscle were analyzed.
RESULTS
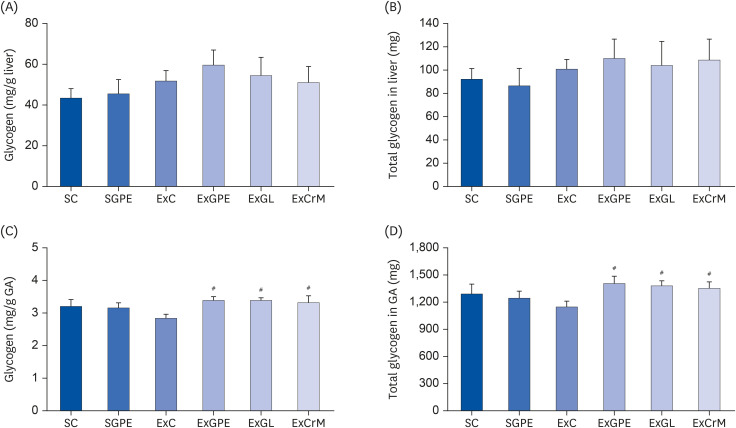
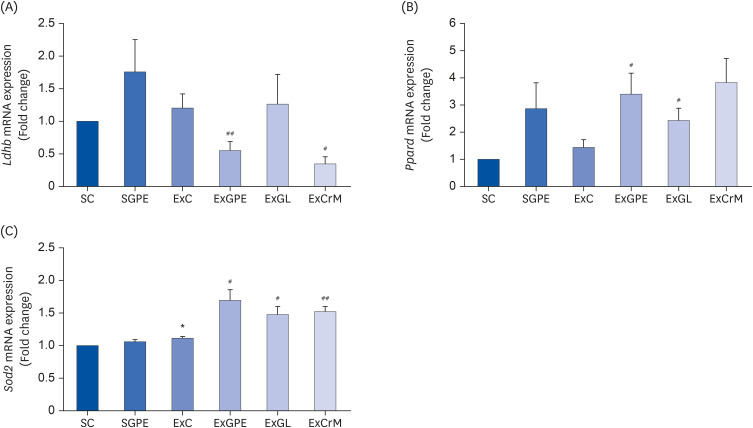
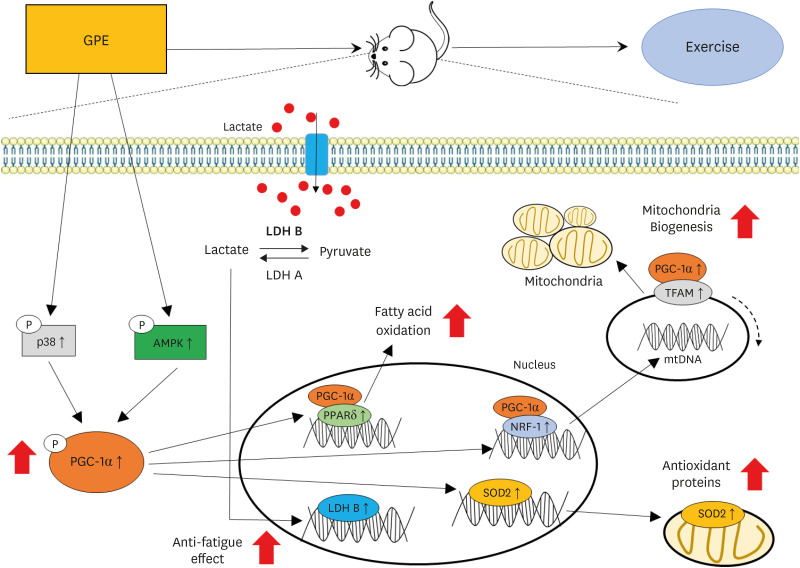
The ExGPE (exercise with 300 mg/kg body weight/day of GPE) mice decreased the fat mass percentage significantly compared to the ExC mice, while the ExGPE showed the greatest lean mass percentage compared to the ExC group. The administration of GPE improved the exercise endurance and capacity in treadmill-trained mice, increased glucose and triglycerides, and decreased the serum creatine kinase and lactate levels after intensive exercise. The muscle glycogen levels were higher in the ExGPE group than the ExC group. GPE increased the level of mitochondrial biogenesis by enhancing the phosphorylation of peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) protein and the mRNA expression of nuclear respiratory factor 1, mitochondrial DNA, peroxisome proliferatoractivated receptor-δ, superoxide dismutase 2, and by decreasing the lactate dehydrogenase B level in the soleus muscle (SOL). GPE also improved PGC-1α activation in the SOL significantly through AMPK/p38 phosphorylation.
CONCLUSIONS
These results showed that GPE supplementation enhances exercise performance and has anti-fatigue activity. In addition, the underlying molecular mechanism was elucidated. Therefore, GPE is a promising candidate for developing functional foods and enhancing the exercise capacity and anti-fatigue activity.
Figure
Reference
-
1. Matsukawa T, Motojima H, Sato Y, Takahashi S, Villareal MO, Isoda H. Upregulation of skeletal muscle PGC-1α through the elevation of cyclic AMP levels by cyanidin-3-glucoside enhances exercise performance. Sci Rep. 2017; 7:44799. PMID: 28317895.2. Safdar A, Little JP, Stokl AJ, Hettinga BP, Akhtar M, Tarnopolsky MA. Exercise increases mitochondrial PGC-1alpha content and promotes nuclear-mitochondrial cross-talk to coordinate mitochondrial biogenesis. J Biol Chem. 2011; 286:10605–10617. PMID: 21245132.3. Wang H, Li C, Wu X, Lou X. Effects of Gynostemma pentaphyllum (Thunb.) Makino polysaccharides supplementation on exercise tolerance and oxidative stress induced by exhaustive exercise in rats. Afr J Agric Res. 2012; 7:2632–2638.4. Lin-Na S, Yong-Xiu S. Effects of polysaccharides from Gynostemma pentaphyllum (Thunb.), Makino on physical fatigue. Afr J Tradit Complement Altern Med. 2014; 11:112–117. PMID: 25371572.5. Shang X, Chao Y, Zhang Y, Lu C, Xu C, Niu W. Immunomodulatory and antioxidant effects of polysaccharides from Gynostemma pentaphyllum Makino in immunosuppressed mice. Molecules. 2016; 21:1085.6. Quan Y, Qian MZ. Effect and mechanism of gypenoside on the inflammatory molecular expression in high-fat induced atherosclerosis rats. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2010; 30:403–406. PMID: 20669679.7. Cai H, Liang Q, Ge G. Gypenoside attenuates beta amyloid-induced inflammation in N9 microglial cells via SOCS1 signaling. Neural Plast. 2016; 2016:6362707. PMID: 27213058.8. Liou CJ, Huang WC, Kuo ML, Yang RC, Shen JJ. Long-term oral administration of Gynostemma pentaphyllum extract attenuates airway inflammation and Th2 cell activities in ovalbumin-sensitized mice. Food Chem Toxicol. 2010; 48:2592–2598. PMID: 20558230.9. Müller C, Gardemann A, Keilhoff G, Peter D, Wiswedel I, Schild L. Prevention of free fatty acid-induced lipid accumulation, oxidative stress, and cell death in primary hepatocyte cultures by a Gynostemma pentaphyllum extract. Phytomedicine. 2012; 19:395–401. PMID: 22381945.10. Gou SH, Huang HF, Chen XY, Liu J, He M, Ma YY, Zhao XN, Zhang Y, Ni JM. Lipid-lowering, hepatoprotective, and atheroprotective effects of the mixture Hong-Qu and gypenosides in hyperlipidemia with NAFLD rats. J Chin Med Assoc. 2016; 79:111–121. PMID: 26842974.11. la Cour B, Mølgaard P, Yi Z. Traditional Chinese medicine in treatment of hyperlipidaemia. J Ethnopharmacol. 1995; 46:125–129. PMID: 7650951.12. Wang M, Wang F, Wang Y, Ma X, Zhao M, Zhao C. Metabonomics study of the therapeutic mechanism of Gynostemma pentaphyllum and atorvastatin for hyperlipidemia in rats. PLoS One. 2013; 8:e78731. PMID: 24223845.13. Lee HS, Lim SM, Jung JI, Kim SM, Lee JK, Kim YH, Cha KM, Oh TK, Moon JM, Kim TY, et al. Gynostemma pentaphyllum extract ameliorates high-fat diet-induced obesity in C57BL/6N mice by upregulating SIRT1. Nutrients. 2019; 11:2475.14. Huyen VT, Phan DV, Thang P, Hoa NK, Ostenson CG. Gynostemma pentaphyllum tea improves insulin sensitivity in type 2 diabetic patients. J Nutr Metab. 2013; 2013:765383. PMID: 23431428.15. Keilhoff G, Esser T, Titze M, Ebmeyer U, Schild L. Gynostemma pentaphyllum is neuroprotective in a rat model of cardiopulmonary resuscitation. Exp Ther Med. 2017; 14:6034–6046. PMID: 29250141.16. Li Y, Lin W, Huang J, Xie Y, Ma W. Anti-cancer effects of Gynostemma pentaphyllum (Thunb.) Makino (Jiaogulan). Chin Med. 2016; 11:43. PMID: 27708693.17. Kao TH, Huang SC, Inbaraj BS, Chen BH. Determination of flavonoids and saponins in Gynostemma pentaphyllum (Thunb.) Makino by liquid chromatography-mass spectrometry. Anal Chim Acta. 2008; 626:200–211. PMID: 18790122.18. Xie Z, Liu W, Huang H, Slavin M, Zhao Y, Whent M, Blackford J, Lutterodt H, Zhou H, Chen P, et al. Chemical composition of five commercial Gynostemma pentaphyllum samples and their radical scavenging, antiproliferative, and anti-inflammatory properties. J Agric Food Chem. 2010; 58:11243–11249. PMID: 20939605.19. Lin CC, Huang PC, Lin JM. Antioxidant and hepatoprotective effects of Anoectochilus formosanus and Gynostemma pentaphyllum . Am J Chin Med. 2000; 28:87–96. PMID: 10794120.20. Liu J, Zhang L, Ren Y, Gao Y, Kang L, Qiao Q. Anticancer and immunoregulatory activity of Gynostemma pentaphyllum polysaccharides in H22 tumor-bearing mice. Int J Biol Macromol. 2014; 69:1–4. PMID: 24832985.21. Attawish A, Chivapat S, Phadungpat S, Bansiddhi J, Techadamrongsin Y, Mitrijit O, Chaorai B, Chavalittumrong P. Chronic toxicity of Gynostemma pentaphyllum . Fitoterapia. 2004; 75:539–551. PMID: 15351107.22. Chi AP, Chen JP, Wang ZZ, Xiong ZY, Li QX. Morphological and structural characterization of a polysaccharide from Gynostemma pentaphyllum Makino and its anti-exercise fatigue activity. Carbohydr Polym. 2008; 74:868–874.23. Yang X, Zhao Y, Yang Y, Ruan Y. Isolation and characterization of immunostimulatory polysaccharide from an herb tea, Gynostemma pentaphyllum Makino. J Agric Food Chem. 2008; 56:6905–6909. PMID: 18636735.24. Ding YJ, Tang KJ, Li FL, Hu QL. Effects of gypenosides from Gynostemma pentaphyllum supplementation on exercise-induced fatigue in mice. Afr J Agric Res. 2010; 5:707–711.25. Tadaishi M, Miura S, Kai Y, Kano Y, Oishi Y, Ezaki O. Skeletal muscle-specific expression of PGC-1α-b, an exercise-responsive isoform, increases exercise capacity and peak oxygen uptake. PLoS One. 2011; 6:e28290. PMID: 22174785.26. Kim YH, Kim SM, Lee JK, Jo SK, Kim HJ, Cha KM, Lim CY, Moon JM, Kim TY, Kim EJ. Efficacy of Gynostemma pentaphyllum extract in anti-obesity therapy. Rec Nat Prod. 2020; 14:116–128.27. Kim YH, Jung JI, Jeon YE, Kim SM, Oh TK, Lee J, Moon JM, Kim TY, Kim EJ. Gynostemma pentaphyllum extract and gypenoside L enhance skeletal muscle differentiation and mitochondrial metabolism by activating the PGC-1α pathway in C2C12 myotubes. Nutr Res Pract. 2021; 15:e45.28. Hearris MA, Hammond KM, Fell JM, Morton JP. Regulation of muscle glycogen metabolism during exercise: implications for endurance performance and training adaptations. Nutrients. 2018; 10:298.29. Jensen J, Rustad PI, Kolnes AJ, Lai YC. The role of skeletal muscle glycogen breakdown for regulation of insulin sensitivity by exercise. Front Physiol. 2011; 2:112. PMID: 22232606.30. Cheng CF, Ku HC, Lin H. PGC-1α as a pivotal factor in lipid and metabolic regulation. Int J Mol Sci. 2018; 19:3447.31. Kang C, Li Ji L. Role of PGC-1α signaling in skeletal muscle health and disease. Ann N Y Acad Sci. 2012; 1271:110–117. PMID: 23050972.32. Wright DC, Han DH, Garcia-Roves PM, Geiger PC, Jones TE, Holloszy JO. Exercise-induced mitochondrial biogenesis begins before the increase in muscle PGC-1α expression. J Biol Chem. 2007; 282:194–199. PMID: 17099248.33. Puigserver P, Rhee J, Lin J, Wu Z, Yoon JC, Zhang CY, Krauss S, Mootha VK, Lowell BB, Spiegelman BM. Cytokine stimulation of energy expenditure through p38 MAP kinase activation of PPARgamma coactivator-1. Mol Cell. 2001; 8:971–982. PMID: 11741533.34. Jäger S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1α. Proc Natl Acad Sci U S A. 2007; 104:12017–12022. PMID: 17609368.35. Wan JJ, Qin Z, Wang PY, Sun Y, Liu X. Muscle fatigue: general understanding and treatment. Exp Mol Med. 2017; 49:e384. PMID: 28983090.36. Facey A, Irving R, Dilworth L. Overview of lactate metabolism and the implications for athletes. J Sports Sci Med. 2013; l:42–46.37. Hirabara SM, Silveira LR, Abdulkader FR, Alberici LC, Procopio J, Carvalho CR, Pithon-Curi TC, Curi R. Role of fatty acids in the transition from anaerobic to aerobic metabolism in skeletal muscle during exercise. Cell Biochem Funct. 2006; 24:475–481. PMID: 16924590.38. DeFronzo RA. Lilly lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes. 1988; 37:667–687. PMID: 3289989.39. Liu Y, Liu C. Antifatigue and increasing exercise performance of Actinidia arguta crude alkaloids in mice. J Food Drug Anal. 2016; 24:738–745. PMID: 28911611.40. Martin WH 3rd, Dalsky GP, Hurley BF, Matthews DE, Bier DM, Hagberg JM, Rogers MA, King DS, Holloszy JO. Effect of endurance training on plasma free fatty acid turnover and oxidation during exercise. Am J Physiol. 1993; 265:E708–E714. PMID: 8238496.41. Horowitz JF, Klein S. Lipid metabolism during endurance exercise. Am J Clin Nutr. 2000; 72:558S–563S. PMID: 10919960.42. Li F, Li J, Li S, Guo S, Li P. Modulatory effects of Chinese herbal medicines on energy metabolism in ischemic heart diseases. Front Pharmacol. 2020; 11:995. PMID: 32719602.43. Kang D, Hamasaki N. Mitochondrial transcription factor A in the maintenance of mitochondrial DNA: overview of its multiple roles. Ann N Y Acad Sci. 2005; 1042:101–108. PMID: 15965051.44. Rowe GC, Jiang A, Arany Z. PGC-1 coactivators in cardiac development and disease. Circ Res. 2010; 107:825–838. PMID: 20884884.45. Nalbandian M, Takeda M. Lactate as a signaling molecule that regulates exercise induced adaptations. Biology (Basel). 2016; 5:38.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Gynostemma pentaphyllum extract and Gypenoside L enhance skeletal muscle differentiation and mitochondrial metabolism by activating the PGC-1α pathway in C2C12 myotubes
- Systematic Evaluation and Meta-Analysis of the Effect of Gynostemma pentaphyllum on Clinical Indexes of Hyperlipidemia
- Treadmill Exercise Improves Motor Function and Short-term Memory by Enhancing Synaptic Plasticity and Neurogenesis in Photothrombotic Stroke Mice
- The influence of different durations of aerobic exercise on fuel utilization, lactate level and antioxidant defense system in trained rats
- Terrible Stent Thrombosis Induced by a Treadmill Test Performed Three Days after Percutaneous Coronary Intervention