Nutr Res Pract.
2023 Aug;17(4):660-669. 10.4162/nrp.2023.17.4.660.
Resveratrol promotes mitochondrial energy metabolism in exerciseinduced fatigued rats
- Affiliations
-
- 1College of Physical Education, Yangzhou University, Yangzhou 225127, China
- KMID: 2545183
- DOI: http://doi.org/10.4162/nrp.2023.17.4.660
Abstract
- BACKGROUND/OBJECTIVES
To investigate the effect and regulatory mechanism of resveratrol supplementation on the mitochondrial energy metabolism of rats with exerciseinduced fatigue.
MATERIALS/METHODS
Forty-eight Sprague-Dawley male rats were divided randomly into a blank control group (C), resveratrol group (R), exercise group (E), and exercise and resveratrol group (ER), with 12 rats in each group. Group ER and group E performed 6-wk swimming training with 5% wt-bearing, 60 min each time, 6 days a wk. Group ER was given resveratrol 50 mg/kg by gavage one hour after exercise; group R was only given resveratrol 50 mg/kg by gavage; group C and group E were fed normally. The same volume of solvent was given by gavage every day.
RESULTS
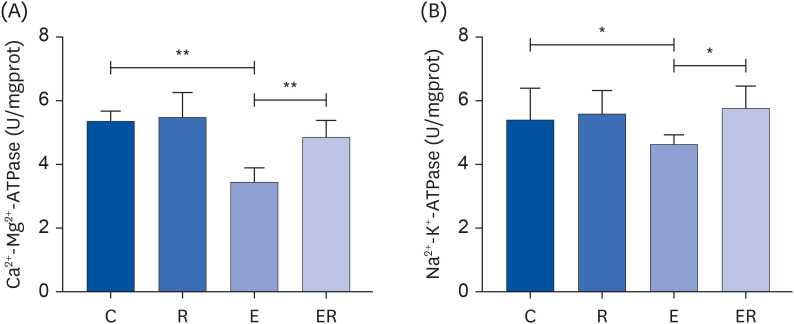
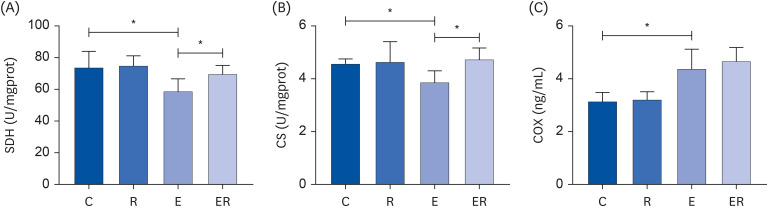
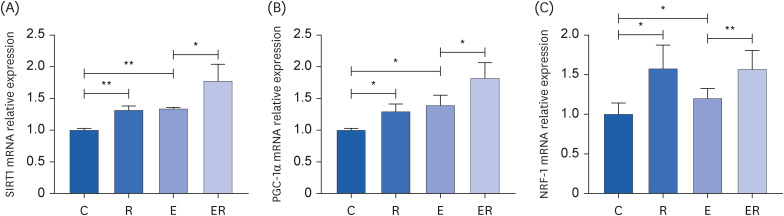
Resveratrol supplementation could reduce the plasma blood urea nitrogen content, creatine kinase activity, and malondialdehyde content in the skeletal muscle, increase the total superoxide dismutase activity in the skeletal muscle, and improve the fatigue state. Resveratrol supplementation could improve the activities of Ca2+ -Mg2+ -ATPase, Na+ -K+ -ATPase, succinate dehydrogenase, and citrate synthase in the skeletal muscle. Furthermore, resveratrol supplementation could up-regulate the sirtuin 1 (SIRT1)-proliferator-activated receptor gamma coactivator-1α (PGC-1α)-nuclear respiratory factor 1 pathway.
CONCLUSIONS
Resveratrol supplementation could promote mitochondrial biosynthesis via the SIRT1/PGC-1α pathway, increase the activity of the mitochondrial energy metabolismrelated enzymes, improve the antioxidant capacity of the body, and promote recovery from exercise-induced fatigue.
Figure
Reference
-
1. Li M, Ning B, Wang T. The mechanism and prevention of mitochondrial injury after exercise. J Physiol Biochem. 2021; 77:215–225. PMID: 33650090.
Article2. Ismaeel A, Holmes M, Papoutsi E, Panton L, Koutakis P. Resistance training, antioxidant status, and antioxidant supplementation. Int J Sport Nutr Exerc Metab. 2019; 29:539–547. PMID: 30859847.
Article3. Corr LD, Field A, Pufal D, Clifford T, Harper LD, Naughton RJ. The effects of cocoa flavanols on indices of muscle recovery and exercise performance: a narrative review. BMC Sports Sci Med Rehabil. 2021; 13:90. PMID: 34391456.
Article4. Solheim SA, Nordsborg NB, Ritz C, Berget J, Kristensen AH, Mørkeberg J. Use of nutritional supplements by Danish elite athletes and fitness customers. Scand J Med Sci Sports. 2017; 27:801–808. PMID: 27264018.
Article5. Galiniak S, Aebisher D, Bartusik-Aebisher D. Health benefits of resveratrol administration. Acta Biochim Pol. 2019; 66:13–21. PMID: 30816367.
Article6. Hou CY, Tain YL, Yu HR, Huang LT. The effects of resveratrol in the treatment of metabolic syndrome. Int J Mol Sci. 2019; 20:535. PMID: 30695995.
Article7. Ma N, Tao H, Du H, Zhao L, Hu Q, Xiao H. Antifatigue effect of functional cookies fortified with mushroom powder (Tricholoma matsutake) in mice. J Food Sci. 2020; 85:4389–4395. PMID: 33159467.
Article8. Fernandes T, Hashimoto NY, Magalhães FC, Fernandes FB, Casarini DE, Carmona AK, Krieger JE, Phillips MI, Oliveira EM. Aerobic exercise training-induced left ventricular hypertrophy involves regulatory MicroRNAs, decreased angiotensin-converting enzyme-angiotensin ii, and synergistic regulation of angiotensin-converting enzyme 2-angiotensin (1-7). Hypertension. 2011; 58:182–189. PMID: 21709209.
Article9. Alway SE, McCrory JL, Kearcher K, Vickers A, Frear B, Gilleland DL, Bonner DE, Thomas JM, Donley DA, Lively MW, et al. Resveratrol enhances exercise-induced cellular and functional adaptations of skeletal muscle in older men and women. J Gerontol A Biol Sci Med Sci. 2017; 72:1595–1606. PMID: 28505227.
Article10. Polley KR, Jenkins N, O’Connor P, McCully K. Influence of exercise training with resveratrol supplementation on skeletal muscle mitochondrial capacity. Appl Physiol Nutr Metab. 2016; 41:26–32. PMID: 26638911.
Article11. Gocmez SS, Gacar N, Utkan T, Gacar G, Scarpace PJ, Tumer N. Protective effects of resveratrol on aging-induced cognitive impairment in rats. Neurobiol Learn Mem. 2016; 131:131–136. PMID: 27040098.
Article12. Wang X, Qu Y, Zhang Y, Li S, Sun Y, Chen Z, Teng L, Wang D. Antifatigue potential activity of Sarcodon imbricatus in acute excise-treated and chronic fatigue syndrome in mice via regulation of Nrf2-mediated oxidative stress. Oxid Med Cell Longev. 2018; 2018:9140896. PMID: 30050662.13. Wang L, Zhang HL, Lu R, Zhou YJ, Ma R, Lv JQ, Li XL, Chen LJ, Yao Z. The decapeptide CMS001 enhances swimming endurance in mice. Peptides. 2008; 29:1176–1182. PMID: 18440669.
Article14. Osman WN, Mohamed S. Standardized Morinda citrifolia L. and Morinda elliptica L. leaf extracts alleviated fatigue by improving glycogen storage and lipid/carbohydrate metabolism. Phytother Res. 2018; 32:2078–2085. PMID: 29993148.
Article15. Zhang G, Mao J, Liang F, Chen J, Zhao C, Yin S, Wang L, Tang Z, Chen S. Modulated expression and enzymatic activities of Darkbarbel catfish, Pelteobagrus vachelli for oxidative stress induced by acute hypoxia and reoxygenation. Chemosphere. 2016; 151:271–279. PMID: 26945243.
Article16. Pirkmajer S, Petrič M, Chibalin AV. The role of AMPK in regulation of Na+,K+-ATPase in skeletal muscle: does the gauge always plug the sink? J Muscle Res Cell Motil. 2021; 42:77–97. PMID: 33398789.
Article17. Wyckelsma VL, Perry BD, Bangsbo J, McKenna MJ. Inactivity and exercise training differentially regulate abundance of Na+-K+-ATPase in human skeletal muscle. J Appl Physiol. 2019; 127:905–920. PMID: 31369327.
Article18. Fernández ÁF, Liu Y, Ginet V, Shi M, Nah J, Zou Z, Zhou A, Posner BA, Xiao G, Tanguy M, et al. Interaction between the autophagy protein Beclin 1 and Na+, K+-ATPase during starvation, exercise, and ischemia. JCI Insight. 2020; 5:e133282. PMID: 31941841.
Article19. Rasheed MR, Tarjan G. Succinate dehydrogenase complex: an updated review. Arch Pathol Lab Med. 2018; 142:1564–1570. PMID: 30289269.
Article20. Jacques M, Kuang J, Bishop DJ, Yan X, Alvarez-Romero J, Munson F, Garnham A, Papadimitriou I, Voisin S, Eynon N. Mitochondrial respiration variability and simulations in human skeletal muscle: the Gene SMART study. FASEB J. 2020; 34:2978–2986. PMID: 31919888.
Article21. Alhindi Y, Vaanholt LM, Al-Tarrah M, Gray SR, Speakman JR, Hambly C, Alanazi BS, Gabriel BM, Lionikas A, Ratkevicius A. Low citrate synthase activity is associated with glucose intolerance and lipotoxicity. J Nutr Metab. 2019; 2019:8594825. PMID: 30944739.
Article22. Reid SN, Park JH, Kim Y, Kwak YS, Jeon BH. In vitro and in vivo effects of fermented oyster-derived lactate on exercise endurance indicators in mice. Int J Environ Res Public Health. 2020; 17:8811. PMID: 33260934.
Article23. Sun J, Zhang C, Kim M, Su Y, Qin L, Dong J, Zhou Y, Ding S. Early potential effects of resveratrol supplementation on skeletal muscle adaptation involved in exercise-induced weight loss in obese mice. BMB Rep. 2018; 51:200–205. PMID: 29519293.
Article24. Vincenzi KL, Maia TP, Delmônego L, Lima AB, Pscheidt LC, Delwing-Dal Magro D, Delwing-de Lima D. Effects of resveratrol on alterations in cerebrum energy metabolism caused by metabolites accumulated in type I citrullinemia in rats. Naunyn Schmiedebergs Arch Pharmacol. 2021; 394:873–884. PMID: 33205249.
Article25. Villareal MO, Matsukawa T, Isoda H. L-citrulline supplementation-increased skeletal muscle PGC-1α expression is associated with exercise performance and increased skeletal muscle weight. Mol Nutr Food Res. 2018; 62:e1701043. PMID: 29797700.
Article26. Vargas-Ortiz K, Pérez-Vázquez V, Macías-Cervantes MH. Exercise and sirtuins: a way to mitochondrial health in skeletal muscle. Int J Mol Sci. 2019; 20:2717. PMID: 31163574.
Article27. Ma S, Feng J, Zhang R, Chen J, Han D, Li X, Yang B, Li X, Fan M, Li C, et al. SIRT1 activation by resveratrol alleviates cardiac dysfunction via mitochondrial regulation in diabetic cardiomyopathy mice. Oxid Med Cell Longev. 2017; 2017:4602715. PMID: 28883902.
Article28. Huang WC, Hsu YJ, Wei L, Chen YJ, Huang CC. Association of physical performance and biochemical profile of mice with intrinsic endurance swimming. Int J Med Sci. 2016; 13:892–901. PMID: 27994494.
Article29. Menzies KJ, Singh K, Saleem A, Hood DA. Sirtuin 1-mediated effects of exercise and resveratrol on mitochondrial biogenesis. J Biol Chem. 2013; 288:6968–6979. PMID: 23329826.
Article30. Chuang YC, Chen SD, Hsu CY, Chen SF, Chen NC, Jou SB. Resveratrol promotes mitochondrial biogenesis and protects against seizure-induced neuronal cell damage in the hippocampus following status epilepticus by activation of the PGC-1α signaling pathway. Int J Mol Sci. 2019; 20:1422–1476. PMID: 30901819.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Resveratrol attenuates aging-induced mitochondrial dysfunction and mitochondria-mediated apoptosis in the rat heart
- The Effects of High Fat Diet and Resveratrol on Mitochondrial Activity of Brown Adipocytes
- Mitochondrial Dysfunction in Diabetic Cardiomyopathy
- Targeting Mitochondrial Dysfunction for the Prevention and Treatment of Metabolic Disease by Bioactive Food Components
- Effects of Resveratrol Supplementation on Oxidative Damage and Lipid Peroxidation Induced by Strenuous Exercise in Rats