Kosin Med J.
2021 Dec;36(2):69-78. 10.7180/kmj.2021.36.2.69.
The Roles of Human Endogenous Retroviruses (HERVs) in Inflammation
- Affiliations
-
- 1Department of Parasitology and Genetics, Kosin University College of Medicine, Busan, Republic of Korea
- KMID: 2524669
- DOI: http://doi.org/10.7180/kmj.2021.36.2.69
Abstract
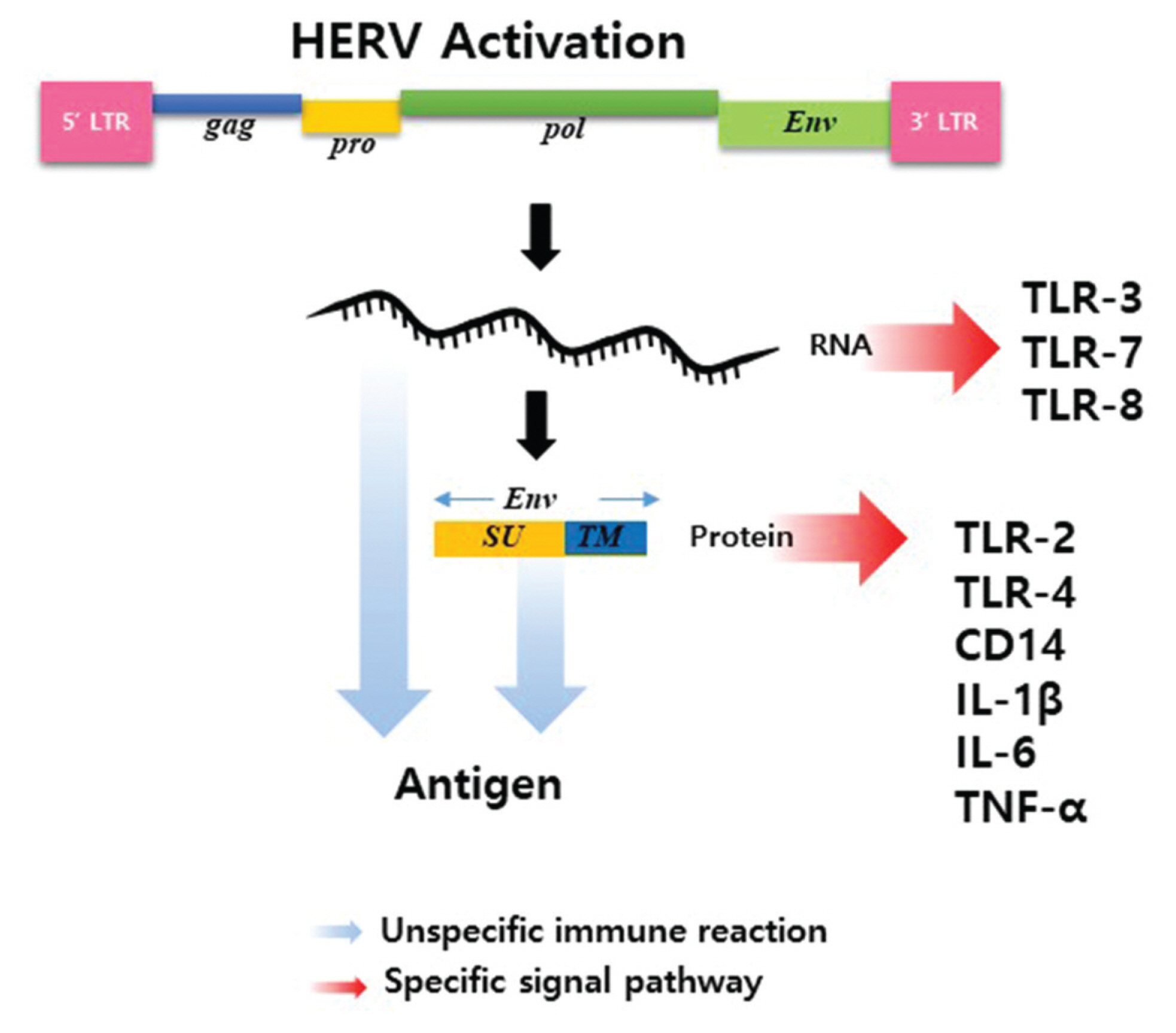
- Human endogenous retroviruses (HERVs) are ancient, currently inactive, and non-infectious due to recombination, deletions, and mutations in the host genome. However, HERV-derived elements are involved in physiological phenomena including inflammatory response. In recent studies, HERV-derived elements were involved directly in various inflammatory diseases including autoimmune diseases such as rheumatoid arthritis (RA), multiple sclerosis, amyotrophic lateral sclerosis (ALS), and Sjogren’s syndrome. Regarding the involvement of HERV-derived elements in inflammation, two possible mechanisms have been proposed. First, HERV-derived elements cause nonspecific innate immune processes. Second, HERV-derived RNA or proteins might stimulate selective signaling mechanisms. However, it is unknown how silent HERV elements are activated in the inflammatory response and what factors and signaling mechanisms are involved with HERV-derived elements. In this review, we introduce HERV-related autoimmune diseases and propose the possible action mechanisms of HERV-derived elements in the inflammatory response at the molecular level.
Figure
Reference
-
1. Ishida T, Obata Y, Ohara N, Matsushita H, Sato S, Uenaka A, et al. Identification of the HERV-K gag antigen in prostate cancer by SEREX using autologous patient serum and its immunogenicity. Cancer Immun. 2008; 8:15.2. Hahn S, Ugurel S, Hanschmann KM, Strobel H, Tondera C, Schadendorf D, et al. Serological response to human endogenous retrovirus K in melanoma patients correlates with survival probability. AIDS Res Hum Retroviruses. 2008; 24:717–23.
Article3. Li M, Radvanyi L, Yin B, Rycaj K, Li J, Chivukula R, et al. Downregulation of Human Endogenous Retrovirus Type K (HERV-K) Viral env RNA in Pancreatic Cancer Cells Decreases Cell Proliferation and Tumor Growth. Clin Cancer Res. 2017; 23:5892–911.
Article4. Wang-Johanning F, Rycaj K, Plummer JB, Li M, Yin B, Frerich K, et al. Immunotherapeutic potential of anti-human endogenous retrovirus-K envelope protein antibodies in targeting breast tumors. J Natl Cancer Inst. 2012; 104:189–210.
Article5. Boese A, Sauter M, Galli U, Best B, Herbst H, Mayer J, et al. Human endogenous retrovirus protein cORF supports cell transformation and associates with the promyelocytic leukemia zinc finger protein. Oncogene. 2000; 19:4328–36.
Article6. Ko EJ, Song KS, Ock MS, Choi YH, Kim S, Kim HS, et al. Expression profiles of Human Endogenous Retrovirus (HERV)-K and HERV-R Env proteins in various cancers. BMB Rep. 2021.
Article7. Rodrigues LS, da Silva Nali LH, Leal COD, Sabino EC, Lacerda EM, Kingdon CC, et al. HERV-K and HERV-W transcriptional activity in myalgic encephalomyelitis/chronic fatigue syndrome. Auto Immun Highlights. 2019; 10:12.
Article8. Dean B, Tawadros N, Scarr E, Gibbons AS. Regionally-specific changes in levels of tumour necrosis factor in the dorsolateral prefrontal cortex obtained postmortem from subjects with major depressive disorder. J Affect Disord. 2010; 120:245–8.
Article9. Portis JL. Perspectives on the role of endogenous human retroviruses in autoimmune diseases. Virology. 2002; 296:1–5.
Article10. Blake DJ, Weir A, Newey SE, Davies KE. Function and genetics of dystrophin and dystrophin-related proteins in muscle. Physiol Rev. 2002; 82:291–329.11. Kury P, Nath A, Creange A, Dolei A, Marche P, Gold J, et al. Human Endogenous Retroviruses in Neurological Diseases. Trends Mol Med. 2018; 24:379–94.12. Saleh A, Macia A, Muotri AR. Transposable Elements, Inflammation, and Neurological Disease. Front Neurol. 2019; 10:894.
Article13. Hung T, Pratt GA, Sundararaman B, Townsend MJ, Chaivorapol C, Bhangale T, et al. The Ro60 autoantigen binds endogenous retroelements and regulates inflammatory gene expression. Science. 2015; 350:455–9.
Article14. Tarlinton R, Wang B, Morandi E, Gran B, Khaiboullin T, Martynova E, et al. Differential Expression of HERV-W in Peripheral Blood in Multiple Sclerosis and Healthy Patients in Two Different Ethnic Groups. Front Pharmacol. 2019; 10:1645.
Article15. Yu HL, Zhao ZK, Zhu F. The role of human endogenous retroviral long terminal repeat sequences in human cancer (Review). Int J Mol Med. 2013; 32:755–62.
Article16. Dolei A, Ibba G, Piu C, Serra C. Expression of HERV Genes as Possible Biomarker and Target in Neurodegenerative Diseases. Int J Mol Sci. 2019; 20.
Article17. Douville R, Liu J, Rothstein J, Nath A. Identification of active loci of a human endogenous retrovirus in neurons of patients with amyotrophic lateral sclerosis. Ann Neurol. 2011; 69:141–51.
Article18. Freimanis G, Hooley P, Ejtehadi HD, Ali HA, Veitch A, Rylance PB, et al. A role for human endogenous retrovirus-K (HML-2) in rheumatoid arthritis: investigating mechanisms of pathogenesis. Clin Exp Immunol. 2010; 160:340–7.
Article19. Rolland A, Jouvin-Marche E, Viret C, Faure M, Perron H, Marche PN. The envelope protein of a human endogenous retrovirus-W family activates innate immunity through CD14/TLR4 and promotes Th1-like responses. J Immunol. 2006; 176:7636–44.
Article20. Wu Z, Mei X, Zhao D, Sun Y, Song J, Pan W, et al. DNA methylation modulates HERV-E expression in CD4+ T cells from systemic lupus erythematosus patients. J Dermatol Sci. 2015; 77:110–6.
Article21. Vassalli P. The pathophysiology of tumor necrosis factors. Annu Rev Immunol. 1992; 10:411–52.
Article22. Groger V, Cynis H. Human Endogenous Retroviruses and Their Putative Role in the Development of Autoimmune Disorders Such as Multiple Sclerosis. Front Microbiol. 2018; 9:265.
Article23. Hishikawa T, Ogasawara H, Kaneko H, Shirasawa T, Matsuura Y, Sekigawa I, et al. Detection of antibodies to a recombinant gag protein derived from human endogenous retrovirus clone 4-1 in autoimmune diseases. Viral Immunol. 1997; 10:137–47.
Article24. Moyes DL, Martin A, Sawcer S, Temperton N, Worthington J, Griffiths DJ, et al. The distribution of the endogenous retroviruses HERV-K113 and HERV-K115 in health and disease. Genomics. 2005; 86:337–41.
Article25. Laska MJ, Troldborg A, Hauge EM, Bahrami S, Stengaard-Pedersen K. Human Endogenous Retroviral Genetic Element With Immunosuppressive Activity in Both Human Autoimmune Diseases and Experimental Arthritis. Arthritis Rheumatol. 2017; 69:398–409.
Article26. Stauffer Y, Marguerat S, Meylan F, Ucla C, Sutkowski N, Huber B, et al. Interferon-alpha-induced endogenous superantigen. a model linking environment and autoimmunity. Immunity. 2001; 15:591–601.27. Clerici M, Fusi ML, Caputo D, Guerini FR, Trabattoni D, Salvaggio A, et al. Immune responses to antigens of human endogenous retroviruses in patients with acute or stable multiple sclerosis. J Neuroimmunol. 1999; 99:173–82.
Article28. Treger RS, Pope SD, Kong Y, Tokuyama M, Taura M, Iwasaki A. The Lupus Susceptibility Locus Sgp3 Encodes the Suppressor of Endogenous Retrovirus Expression SNERV. Immunity. 2019; 50:334–47.e9.
Article29. Mikhalkevich N, O’Carroll IP, Tkavc R, Lund K, Sukumar G, Dalgard CL, et al. Response of human macrophages to gamma radiation is mediated via expression of endogenous retroviruses. PLoS Pathog. 2021; 17:e1009305.
Article30. Morozov VA, Morozov AV, Semaan M, Denner J. Single mutations in the trans-membrane envelope protein abrogate the immunosuppressive property of HIV-1. Retrovirology. 2012; 9:67.
Article31. Beiting DP, Bliss SK, Schlafer DH, Roberts VL, Appleton JA. Interleukin-10 limits local and body cavity inflammation during infection with muscle-stage Trichinella spiralis. Infect Immun. 2004; 72:3129–37.32. Dembny P, Newman AG, Singh M, Hinz M, Szczepek M, Kruger C, et al. Human endogenous retrovirus HERV-K(HML-2) RNA causes neurodegeneration through Toll-like receptors. JCI Insight. 2020; 5.
Article33. Ariza ME, Williams MV. A human endogenous retrovirus K dUTPase triggers a TH1, TH17 cytokine response: does it have a role in psoriasis? J Invest Dermatol. 2011; 131:2419–27.
Article34. Kawai T, Akira S. TLR signaling. Semin Immunol. 2007; 19:24–32.
Article35. Ko EJ, Ock MS, Choi YH, Iovanna JL, Mun S, Han K, et al. Human Endogenous Retrovirus (HERV)-K env Gene Knockout Affects Tumorigenic Characteristics of nupr1 Gene in DLD-1 Colorectal Cancer Cells. Int J Mol Sci. 2021; 22.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Human Endogenous Retroviruses: Friends and Foes in Urology Clinics
- Expression and Packaging of a Human Endogenous Retrovirus-K Genomic DNA Clone
- Genomic Features of Retroelements and Implications for Human Disease
- Transposable Elements: No More 'Junk DNA'
- Comparison of the age-related porcine endogenous retrovirus (PERV) expression using duplex RT-PCR