J Bacteriol Virol.
2006 Dec;36(4):237-245. 10.4167/jbv.2006.36.4.237.
Expression and Packaging of a Human Endogenous Retrovirus-K Genomic DNA Clone
- Affiliations
-
- 1Department of Molecular Biology and Microbiology Tufts University School of Medicine 136 Harrison Avenue, Boston, MA 02111, USA. ymhalee@yonsei.ac.kr
- KMID: 2055032
- DOI: http://doi.org/10.4167/jbv.2006.36.4.237
Abstract
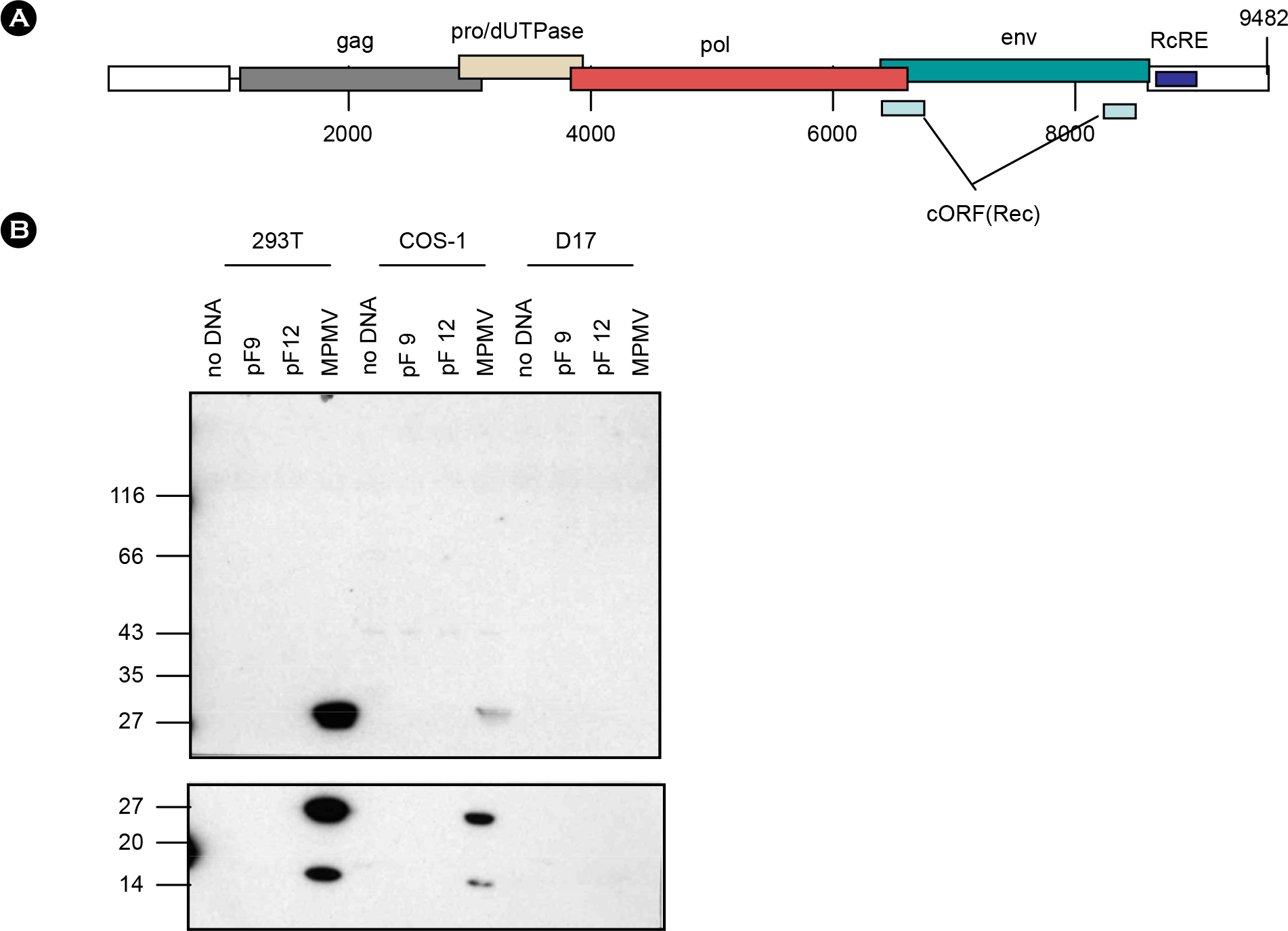
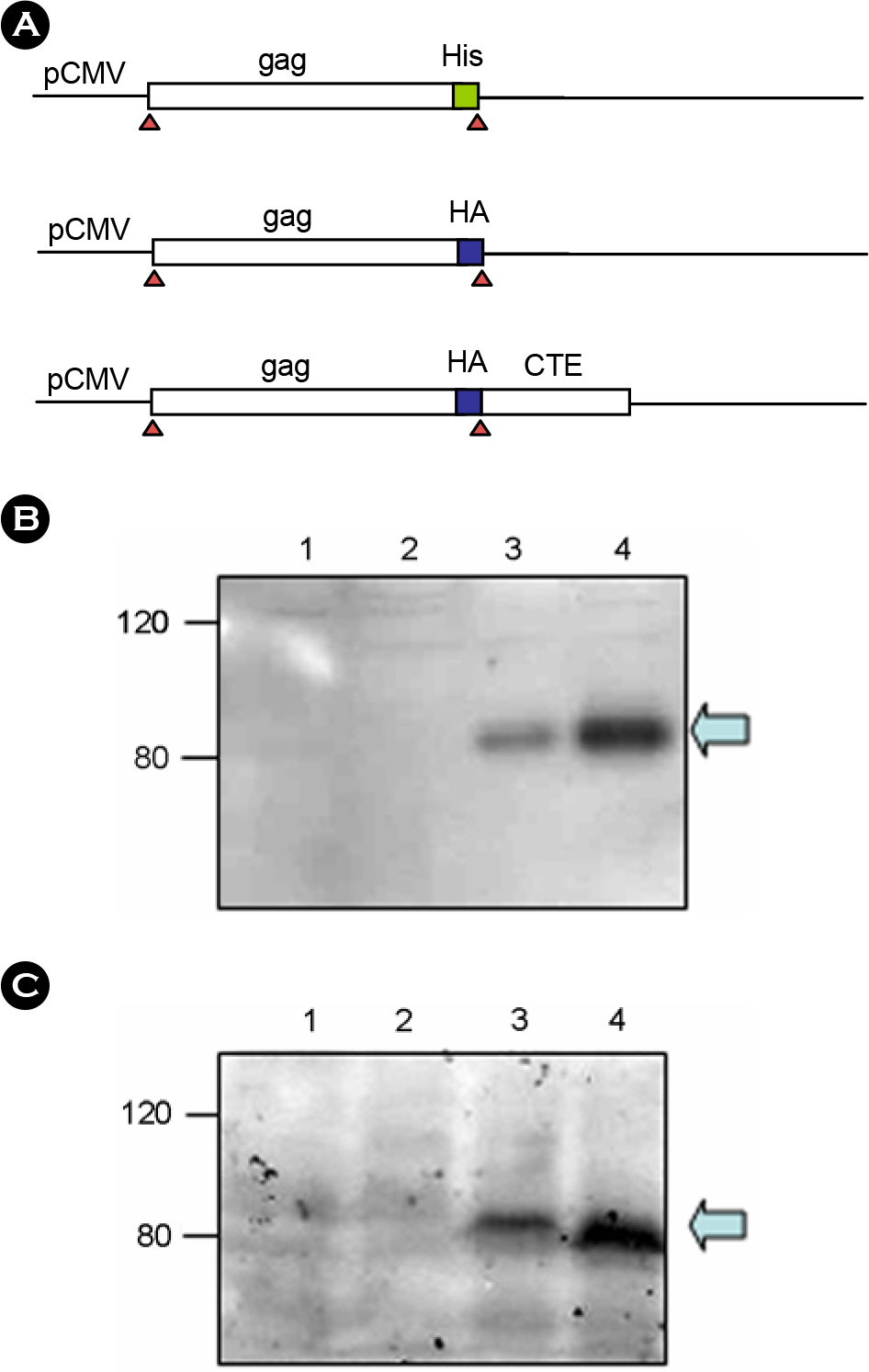
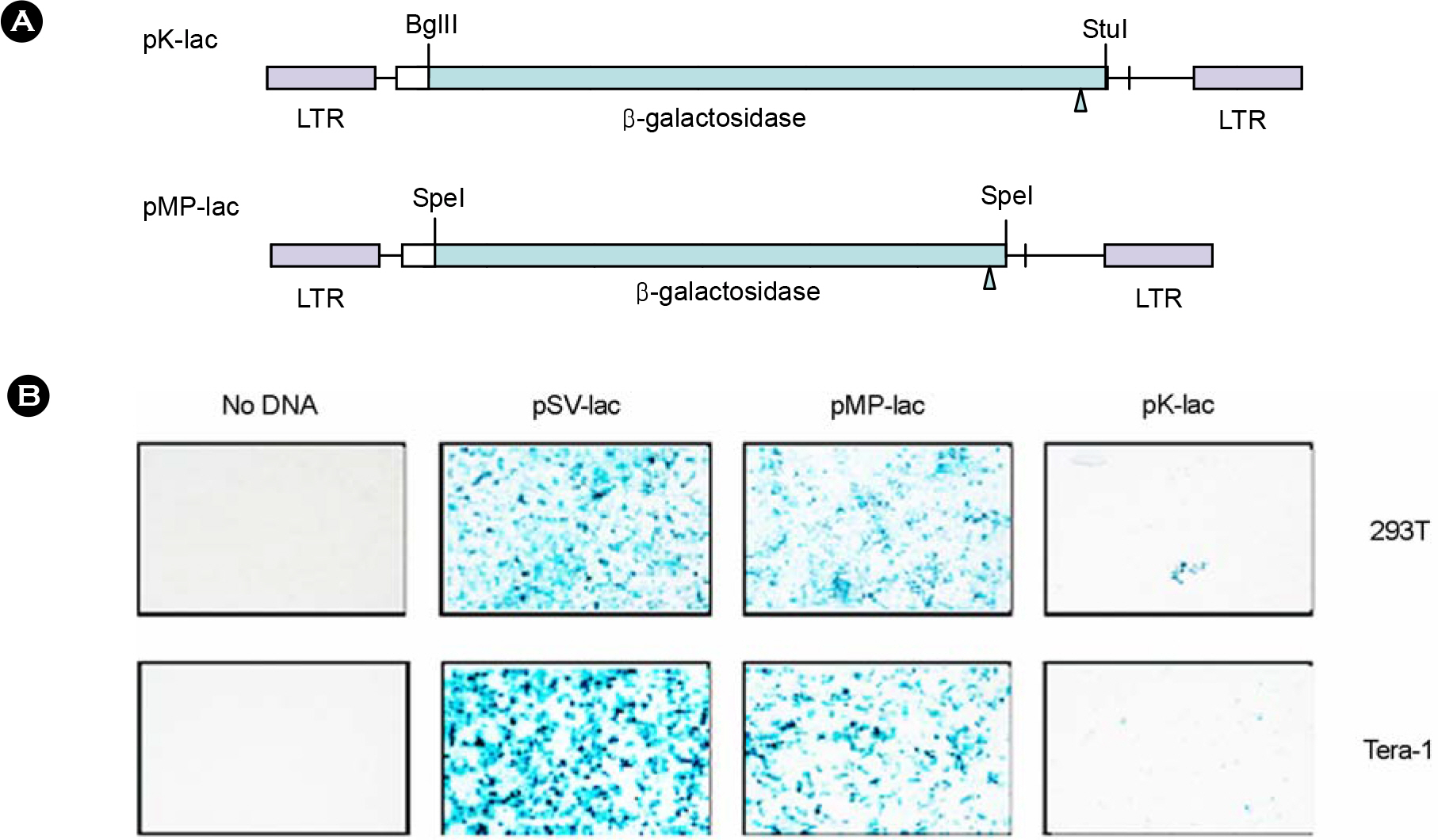
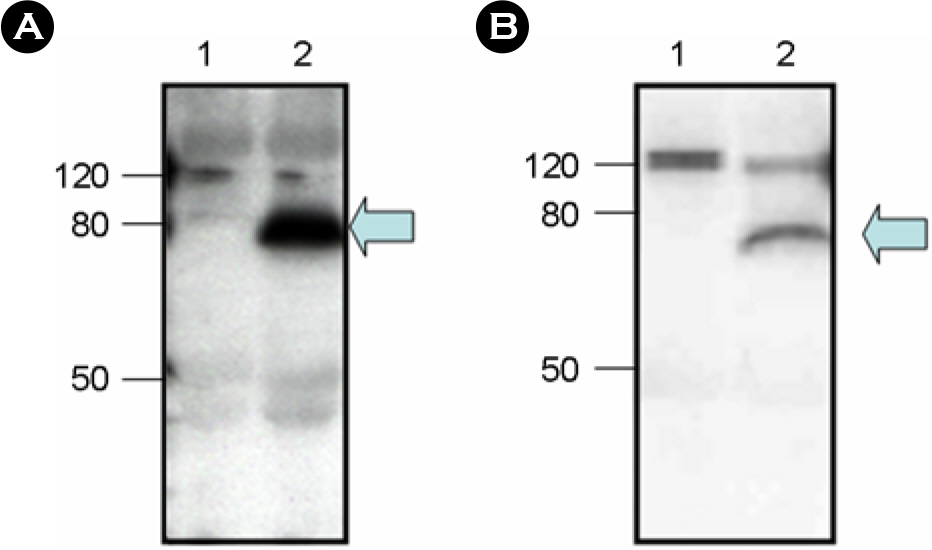
- Human contains large number of human endogenous retroviruses (HERVs) in its genome. One of the HERV families, HERV-K, entered human genome most recently and includes many members with full-length intact proviruses. Normally, these proviruses do not express but infrequently they seem to express in cancers or autoimmune disease patients. To investigate expression mechanisms of these endogenous retroviruses, a DNA copy of HERV-K was cloned and its expression was studied. The transfection of the full-length clone into human cell lines did not produce any detectable viral capsid protein, Gag, and the transcription from its own promoter in LTR was extremely poor. The transcription was less than 10 percent compare to the exogenous retrovirus. However, when the Gag coding region was cloned under CMV promoter, Gag could be expressed efficiently and secreted as particles, probably virus like particles. The efficient expression also required a nuclear export signal. The expressed Gag could also package its own genomic RNA. These results indicate that the LTR of HERV-K is normally not active but its genes have a potential to express and possibly produce infectious particles.
Keyword
MeSH Terms
Figure
Reference
-
References
1). Bannert N, Kurth R. Retroelements and the human genome: new perspectives on an old relation. Proc Natl Acad Sci U S A. 101(Suppl 2):14572–14579. 2004.
Article2). Barbulescu M, Turner G, Seaman MI, Deinard AS, Kidd KK, Lenz J. Many human endogenous retro-virus K (HERV-K) proviruses are unique to humans. Curr Biol. 9:861–868. 1999.
Article3). Belshaw R, Dawson AL, Woolven-Allen J, Redding J, Burt A, Tristem M. Genomewide screening reveals high levels of insertional polymorphism in the human endogenous retrovirus family HERV-K (HML2): implications for present-day activity. J Virol. 79:12507–12514. 2005.4). Berkhout B, Jebbink M, Zsiros J. Identification of an active reverse transcriptase enzyme encoded by a human endogenous HERV-K retrovirus. J Virol. 73:2365–2375. 1999.
Article5). Boller K, Konig H, Sauter M, Mueller-Lantzsch N, Lower R, Lower J, Kurth R. Evidence that HERV-K is the endogenous retrovirus sequence that codes for the human teratocarcinoma-derived retrovirus HTDV. Virology. 196:349–353. 1993.
Article6). Bray M, Prasad S, Dubay JW, Hunter E, Jeang KT, Rekosh D, Hammarskjold ML. A small element from the Mason-Pfizer monkey virus genome makes human immunodeficiency virus type 1 expression and replication Rev-independent. Proc Natl Acad Sci U S A. 91:1256–1260. 1994.7). Dewannieux M, Blaise S, Heidmann T. Identification of a functional envelope protein from the HERV-K family of human endogenous retroviruses. J Virol. 79:15573–15577. 2005.
Article8). Ernst RK, Bray M, Rekosh D, Hammarskjold ML. A structured retroviral RNA element that mediates nucleocytoplasmic export of intron-containing RNA. Mol Cell Biol. 17:135–144. 1997.
Article9). Kitamura Y, Ayukawa T, Ishikawa T, Kanda T, Yoshiike K. Human endogenous retrovirus K10 encodes a functional integrase. J Virol. 70:3302–3306. 1996.
Article10). Kovalskaya E, Buzdin A, Gogvadze E, Vinogradova T, Sverdlov E. Functional human endogenous retro-viral LTR transcription start sites are located between the R and U5 regions. Virology. 346:373–378. 2006.
Article11). Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, Fitz-Hugh W, Funke R, Gage D, Harris K, Heaford A, Howland J, Kann L, Lehoczky J, LeVine R, McEwan P, McKernan K, Meldrim J, Mesirov JP, Miranda C, Morris W, Naylor J, Raymond C, Rosetti M, Santos R, Sheridan A, Sougnez C, Stange-Thomann N, Stojanovic N, Subramanian A, Wyman D, Rogers J, Sulston J, Ainscough R, Beck S, Bentley D, Burton J, Clee C, Carter N, Coulson A, Deadman R, Deloukas P, Dunham A, Dunham I, Durbin R, French L, Grafham D, Gregory S, Hubbard T, Humphray S, Hunt A, Jones M, Lloyd C, McMurray A, Matthews L, Mercer S, Milne S, Mullikin JC, Mungall A, Plumb R, Ross M, Shownkeen R, Sims S, Waterston RH, Wilson RK, Hillier LW, McPherson JD, Marra MA, Mardis ER, Fulton LA, Chinwalla AT, Pepin KH, Gish WR, Chissoe SL, Wendl MC, Delehaunty KD, Miner TL, Delehaunty A, Kramer JB, Cook LL, Fulton RS, Johnson DL, Minx PJ, Clifton SW, Hawkins T, Branscomb E, Predki P, Richardson P, Wenning S, Slezak T, Doggett N, Cheng JF, Olsen A, Lucas S, Elkin C, Uberbacher E, Frazier M, et al. Initial sequencing and analysis of the human genome. Nature. 409:860–921. 2001.
Article12). Lavie L, Kitova M, Maldener E, Meese E, Mayer J. CpG methylation directly regulates transcriptional activity of the human endogenous retrovirus family HERV-K (HML-2). J Virol. 79:876–883. 2005.13). Lower R, Boller K, Hasenmaier B, Korbmacher C, Muller-Lantzsch N, Lower J, Kurth R. Identification of human endogenous retroviruses with complex mRNA expression and particle formation. Proc Natl Acad Sci U S A. 90:4480–4484. 1993.
Article14). Lower R, Lower J, Kurth R. The viruses in all of us: characteristics and biological significance of human endogenous retrovirus sequences. Proc Natl Acad Sci U S A. 93:5177–5184. 1996.
Article15). Lower R, Tonjes RR, Korbmacher C, Kurth R, Lower J. Identification of a Rev-related protein by analysis of spliced transcripts of the human endogenous retroviruses HTDV/HERV-K. J Virol. 69:141–149. 1995.
Article16). Magin-Lachmann C, Hahn S, Strobel H, Held U, Lower J, Lower R. Rec (formerly Corf) function requires interaction with a complex, folded RNA structure within its responsive element rather than binding to a discrete specific binding site. J Virol. 75:10359–10371. 2001.
Article17). Magin C, Lower R, Lower J. cORF and RcRE, the Rev/Rex and RRE/RxRE homologues of the human endogenous retrovirus family HTDV/HERV-K. J Virol. 73:9496–9507. 1999.
Article18). Mayer J, Meese E, Mueller-Lantzsch N. Human endogenous retrovirus K homologous sequences and their coding capacity in Old World primates. J Virol. 72:1870–1875. 1998.
Article19). Muster T, Waltenberger A, Grassauer A, Hirschl S, Caucig P, Romirer I, Fodinger D, Seppele H, Schanab O, Magin-Lachmann C, Lower R, Jansen B, Pehamberger H, Wolff K. An endogenous retrovirus derived from human melanoma cells. Cancer Res. 63:8735–8741. 2003.20). Ono M, Yasunaga T, Miyata T, Ushikubo H. Nucleotide sequence of human endogenous retrovirus genome related to the mouse mammary tumor virus genome. J Virol. 60:589–598. 1986.
Article21). Tonjes RR, Boller K, Limbach C, Lugert R, Kurth R. Characterization of human endogenous retrovirus type K virus-like particles generated from recombinant baculoviruses. Virology. 233:280–291. 1997.
Article22). Tonjes RR, Czauderna F, Kurth R. Genome-wide screening, cloning, chromosomal assignment, and expression of full-length human endogenous retrovirus type K. J Virol. 73:9187–9195. 1999.
Article23). Tonjes RR, Limbach C, Lower R, Kurth R. Expression of human endogenous retrovirus type K envelope glycoprotein in insect and mammalian cells. J Virol. 71:2747–2756. 1997.
Article24). Towler EM, Gulnik SV, Bhat TN, Xie D, Gustschina E, Sumpter TR, Robertson N, Jones C, Sauter M, Mueller-Lantzsch N, Debouck C, Erickson JW. Functional characterization of the protease of human endogenous retrovirus, K10: can it complement HIV-1 protease? Biochemistry. 37:17137–17144. 1998.
Article25). Turner G, Barbulescu M, Su M, Jensen-Seaman MI, Kidd KK, Lenz J. Insertional polymorphisms of full-length endogenous retroviruses in humans. Curr Biol. 11:1531–1535. 2001.
Article26). Wodrich H, Schambach A, Krausslich HG. Multiple copies of the Mason-Pfizer monkey virus constitutive RNA transport element lead to enhanced HIV-1 Gag expression in a context-dependent manner. Nucleic Acids Res. 28:901–910. 2000.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Characterization of Clones of Human Cell Line Infected with Porcine Endogenous Retrovirus (PERV) from Porcine Cell Line, PK-15
- Human genomic DNA isolation and chromosomal localization of fetal brain cDNA (FB174)
- Detection of Human Cytomegalovirus (HCMV) and Porcine Endogenous Retrovirus (PERV) with One Step Extraction Method
- Transposable Elements: No More 'Junk DNA'
- Analysis of the global gene expression profiles in genomic instability-induced cervical cancer cells