Infect Chemother.
2009 Feb;41(1):1-8. 10.3947/ic.2009.41.1.1.
Characterization of Clones of Human Cell Line Infected with Porcine Endogenous Retrovirus (PERV) from Porcine Cell Line, PK-15
- Affiliations
-
- 1Department of Microbiology and Immunology, Seoul National University College of Medicine, Seoul, Korea. hesss@snu.ac.kr
- 2Hamchoon Institute of Fertility & Genetics, Hamchoon Womens Clinic, Seoul, Korea.
- KMID: 2170243
- DOI: http://doi.org/10.3947/ic.2009.41.1.1
Abstract
-
BACKGROUND: Porcine endogenous retroviruses (PERVs) form part of the chromosomes of all pigs. Since they can be produced as infectious virion and infect human cells, safety issues on PERVs infection to human are still controversial and is one of main hurdles of xenotransplantation using pig cells or organs. It has been reported that the established porcine cell line, PK-15, produces PERVs and can infect the human cell lines. Therefore, clonal analysis on human cell line infected with PERV is a prerequisite to characterize the infectivity to human cells and to investigate the harmfulness of PERVs to human.
MATERIALS AND METHODS
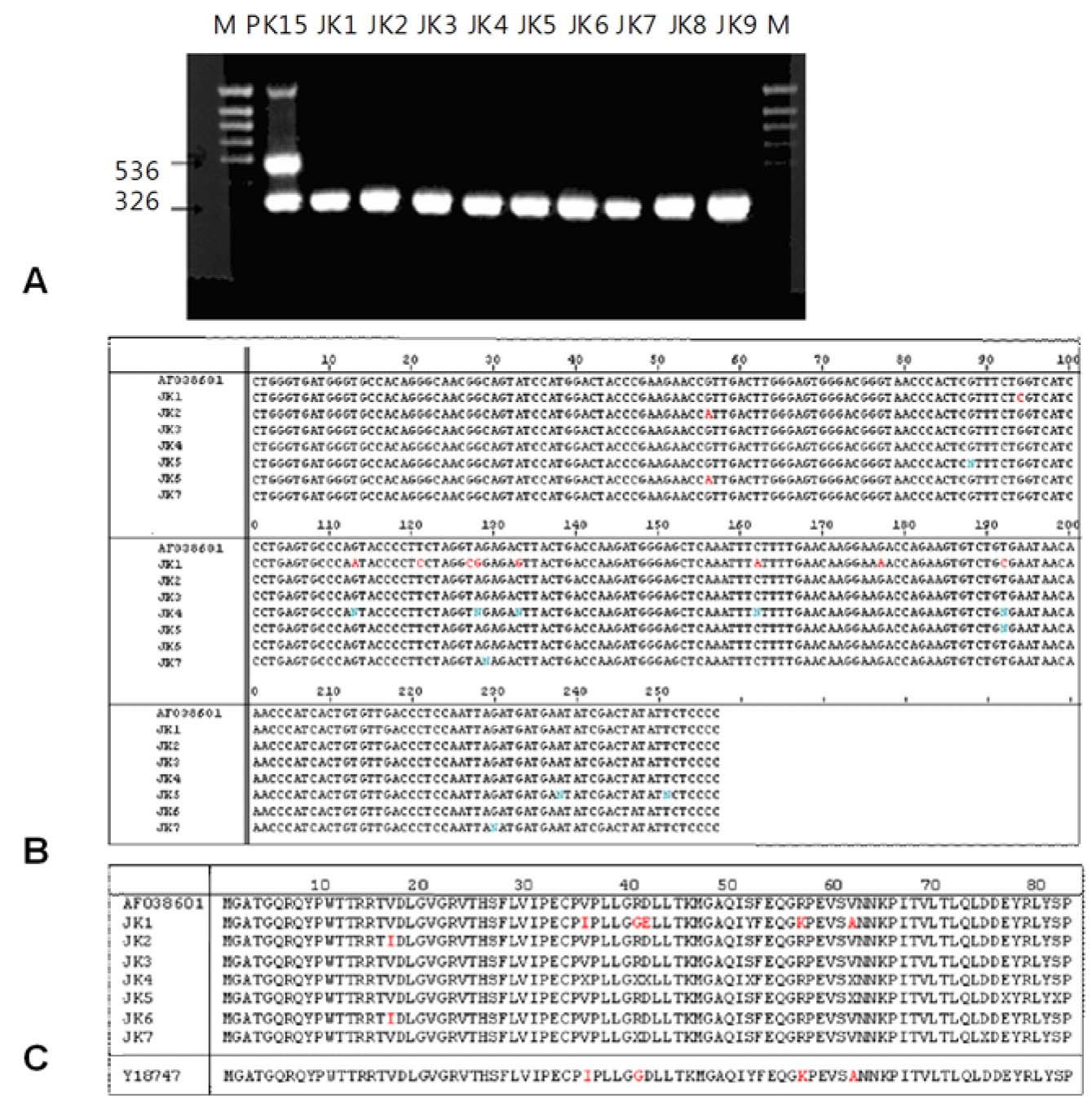
For the characterization of PERV that originates from porcine cell line, PK-15, full length PERV cloning from genomic DNA of PK-15 was performed and partial sequences of both ends were achieved. Cell clones from human cell line, 293, persistently infected with PERVs from PK-15 were established by the method of limiting dilution. Nested PCR and direct sequencing of PCR products in each clone were carried out so as to confirm the PERV genomes in each clone. The growth rate of each clone was checked using cell counting and 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay, the infectivity by reverse transcriptase (RT) assay, and genetic analysis by karyotyping.
RESULTS
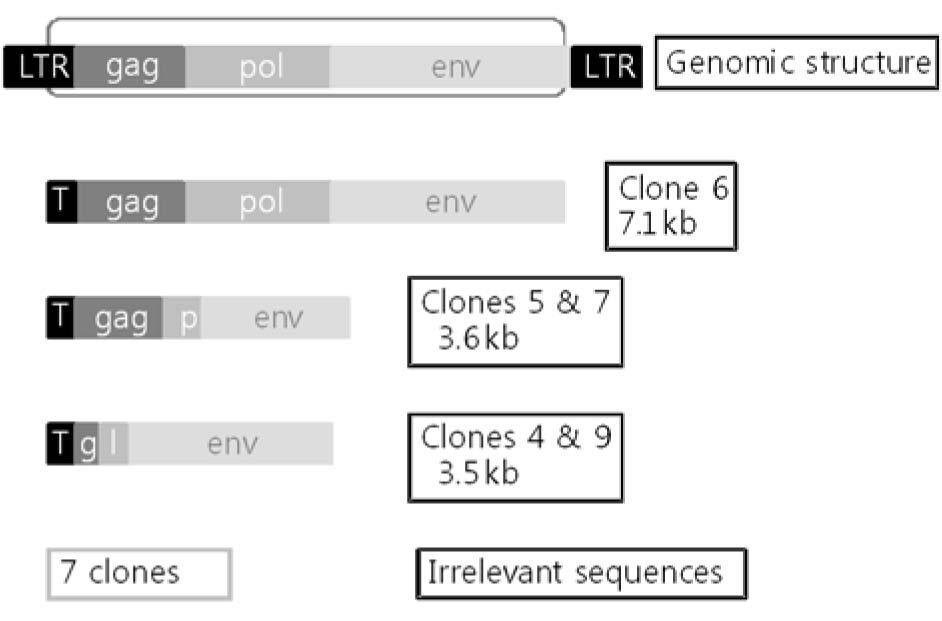
A total of 12 genomic PERV clones could be retrieved; 1 with full length, 4 with defective forms, and others with irrelevant sequences. Intact PERV was thought to be able to infect 293 and the PERV-infected cell clones were selected by limiting dilution. PCR results confirmed that nine cell clones were infected with PERV, and sequence alignment data on PCR products of pol region from PK-15 and human cell clones with PERV showed very similar results. Cell counting and MTT assay for growth kinetics of each clone indicated that two clones showed reduced growth rate. However, it was difficult to verify the effect of PERV infection on the cell growth because of the presence of many genetic alterations in 293 parental cells. No RT activities were detected in the culture supernatant from PERV-infected 293 cell clones.
CONCLUSION
The sequences of PERVs were detected in human cell clones after PERV infection, but PERV virions could not be detected from the culture supernatant by RT assay.
MeSH Terms
Figure
Cited by 1 articles
-
No Evidence of the Productive Replication of Porcine Endogenous Retrovirus (PERV) from SNU Miniature Pigs in Human Cell Line
Jung Heon Kim, Eun-Suk Jung, Chung-Gyu Park, Sang Joon Kim, Eung Soo Hwang
Infect Chemother. 2010;42(3):175-180. doi: 10.3947/ic.2010.42.3.175.
Reference
-
1. Sachs DH, Sykes M, Robson SC, Cooper DK. Xenotransplantation. Adv Immunol. 2001. 79:129–223.
Article2. Denner J. Recombinant porcine endogenous retroviruses (PERV-A/C): a new risk for xenotransplantation? Arch Virol. 2008. 153:1421–1426.
Article3. Le Tissier P, Stoye JP, Takeuchi Y, Patience C, Weiss RA. Two sets of human-tropic pig retrovirus. Nature. 1997. 389:681–682.
Article4. Patience C, Switzer WM, Takeuchi Y, Griffiths DJ, Goward ME, Heneine W, Stoye JP, Weiss RA. Multiple groups of novel retroviral genomes in pigs and related species. J Virol. 2001. 75:2771–2775.
Article5. Ericsson T, Oldmixon B, Blomberg J, Rosa M, Patience C, Andersson G. Identification of novel porcine endogenous betaretrovirus sequences in miniature swine. J Virol. 2001. 75:2765–2770.
Article6. Mang R, Maas J, Chen X, Goudsmit J, van Der Kuyl AC. Identification of novel type C porcine endogenous retrovirus: evidence that copy number of endogenous retrovirus increases during host inbreeding. J Gen Virol. 2001. 82:1829–1834.
Article7. Denner J. Immunosuppression by retroviruses: implications for xenotransplantation. Ann N Y Acad Sci. 1998. 862:75–86.
Article8. Armstrong JA, Porterfield JS, De Madrid AT. C-type virus particles in pig kidney cell lines. J Gen Virol. 1971. 10:195–198.
Article9. Patience C, Takeuchi Y, Weiss RA. Infection of human cells by an endogenous retrovirus of pigs. Nat Med. 1997. 3:282–286.
Article10. Bösch S, Arnauld C, Jestin A. Study of full-length porcine endogenous retrovirus genomes with envelope gene polymorphism in a specific-pathogen-free Large White swine herd. J Virol. 2000. 74:8575–8581.
Article11. Nyberg SL, Hibbs JR, Hardin JA, Germer JJ, Platt JL, Paya CV, Wiesner RH. Influence of human fulminant hepatic failure sera on endogenous retroviral expression in pig hepatocytes. Liver Transpl. 2000. 6:76–84.
Article12. Yu P, Zhang L, Li SF, Cheng JQ, Lu YR, Zeng YZ, Li YP, Bu H. A rapid method for detection of the copy number of porcine endogenous retrovirus in swine. J Rapid Methods Autom Microbiol. 2007. 15:199–205.
Article13. Specke V, Rubant S, Denner J. Productive infection of human primary cells and cell lines with porcine endogenous retroviruses. Virology. 2001. 285:177–180.
Article14. Wilson CA, Wong S, VanBrocklin M, Federspiel MJ. Extended analysis of the in vitro tropism of porcine endogenous retrovirus. J Virol. 2000. 74:49–56.
Article15. Martin U, Winkler ME, Id M, Radeke H, Arseniev L, Takeuchi Y, Simon AR, Patience C, Haverich A, Steinhoff G. Productive infection of primary human endothelial cells by pig endogenous retrovirus (PERV). Xenotransplantation. 2000. 7:138–142.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Construction of the Porcine Endogenous Retrovirus Envelope Glycoprotein A and B Specific Antibody
- No Evidence of the Productive Replication of Porcine Endogenous Retrovirus (PERV) from SNU Miniature Pigs in Human Cell Line
- Analysis of env Subtypes of Porcine Endogenous Retrovirus in SNU Miniature Pigs
- Molecular Characterization of Porcine Endogenous Retrovirus gag Genes from Pigs in Korea
- Analysis of swine leukocyte antigen class I gene profiles and porcine endogenous retrovirus viremia level in a transgenic porcine herd inbred for xenotransplantation research