Infect Chemother.
2010 Jun;42(3):175-180. 10.3947/ic.2010.42.3.175.
No Evidence of the Productive Replication of Porcine Endogenous Retrovirus (PERV) from SNU Miniature Pigs in Human Cell Line
- Affiliations
-
- 1Department of Microbiology and Immunology, Seoul National University College of Medicine, Seoul, Korea. hesss@snu.ac.kr
- 2Department of Surgery, Seoul National University College of Medicine, Seoul, Korea.
- 3Institute of Endemic Diseases, Seoul National University Medical Research Center, Seoul, Korea.
- 4Transplantation Research Institute, Seoul National University Medical Research Center, Seoul, Korea.
- 5Seoul National University Cancer Research Institute, Seoul, Korea.
- 6Center for Animal Resource Development, Seoul National University College of Medicine, Seoul, Korea.
- 7Xenotransplantation Research Center, Seoul National University College of Medicine, Seoul, Korea.
- 8BK21 Division of Human Life Science, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2170306
- DOI: http://doi.org/10.3947/ic.2010.42.3.175
Abstract
- BACKGROUND
The presence of porcine endogenous retrovirus (PERV) has been considered as one of the main hurdles to transplant pig's organs or tissues to human beings. There has been no report that PERV infection is associated with human diseases. Because pigs have their own characteristics of PERV according to pig strain, it is necessary to analyze the infectivity of PERV from SNU miniature pig to human cells for future utilization as a transplantation donor.
MATERIALS AND METHODS
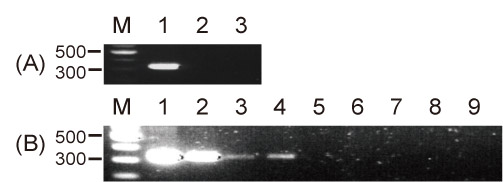
Human cell lines were infected with culture supernatant from porcine cell line or immunomodulator-stimulated peripheral blood mononuclear cells (PBMC) of SNU miniature pigs. They were also co-cultured with PBMC or islet cells of SNU miniature pigs. The presence of PERV genes and general pig marker gene in cells was determined by nested PCR with primer set for PERV pol and pig mitochondrial cytochrome oxidase II (COII), respectively.
RESULTS
Infection test with the culture supernatant from PBMC of SNU miniature pigs showed that PERV pol but not COII was detected only in a few cases, but there was no uniform infection pattern in scope of stimulators and cell types. PERV pol was not demonstrated in co-cultures of human cell line with PBMC or islet cells from SNU miniature pigs after 80 days of co-cultures.
CONCLUSIONS
In vitro infectivity test suggests that PERV from SNU miniature pig might not replicate productively in human cell lines although it could infect human cells and integrate into chromosome.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Analysis of
env Subtypes of Porcine Endogenous Retrovirus in SNU Miniature Pigs
Moa Sa, Chung-Gyu Park, Eung-Soo Hwang
J Bacteriol Virol. 2014;44(1):75-83. doi: 10.4167/jbv.2014.44.1.75.
Reference
-
1. Sachs DH, Sykes M, Robson SC, Cooper DK. Xenotransplantation. Adv Immunol. 2001; 79:129–223.
Article2. Patience C, Takeuchi Y, Weiss RA. Infection of human cells by an endogenous retrovirus of pigs. Nat Med. 1997; 3:282–286.
Article3. Tacke SJ, Kurth R, Denner J. Porcine endogenous retroviruses inhibit human immune cell function: risk for xenotransplantation? Virology. 2000; 268:87–93.
Article4. Oldmixon BA, Wood JC, Ericsson TA, Wilson CA, White-Scharf ME, Andersson G, Greenstein JL, Schuurman HJ, Patience C. Porcine endogenous retrovirus transmission characteristics of an inbred herd of miniature swine. J Virol. 2002; 76:3045–3048.
Article5. Valdes-Gonzalez R, Dorantes LM, Bracho-Blanchet E, Rodríguez-Ventura A, White DJ. No evidence of porcine endogenous retrovirus in patients with type 1 diabetes after long-term porcine islet xenotransplantation. J Med Virol. 2010; 82:331–334.
Article6. Tacke SJ, Specke V, Denner J. Differences in release and determination of subtype of porcine endogenous retroviruses produced by stimulated normal pig blood cells. Intervirology. 2003; 46:17–24.
Article7. Kim HI, Lee SY, Jin SM, Kim KS, Yu JE, Yeom SC, Yoon TW, Kim JH, Ha J, Park CG, Kim SJ. Parameters for successful pig islet isolation as determined using 68 specific-pathogen-free miniature pigs. Xenotransplantation. 2009; 16:11–18.
Article8. Deng Y, Tuch BE, Rawlinson WD. Transmission of porcine endogenous retroviruses in severe combined immunodeficient mice xenotransplanted with fetal porcine pancreatic cells. Transplantation. 2000; 70:1010–1016.9. Armstrong JA, Porterfield JS, De Madrid AT. C-type virus particles in pig kidney cell lines. J Gen Virol. 1971; 10:195–198.
Article10. Le Tissier P, Stoye JP, Takeuchi Y, Patience C, Weiss RA. Two sets of human-tropic pig retrovirus. Nature. 1997; 389:681–682.
Article11. Takeuchi Y, Patience C, Magre S, Weiss RA, Banerjee PT, Le Tissier P, Stoye JP. Host range and interference studies of three classes of pig endogenous retrovirus. J Virol. 1998; 72:9986–9991.
Article12. Martin U, Winkler ME, Id M, Radecke H, Arseniev L, Grotelüschen R, Simon AR, Steinhoff G. Transmission of pig endogenous retrovirus to primary human cells. Transplant Proc. 2000; 32:115–117.
Article13. Martin U, Winkler ME, Id M, Radeke H, Arseniev L, Takeuchi Y, Simon AR, Patience C, Haverich A, Steinhoff G. Productive infection of primary human endothelial cells by pig endogenous retrovirus (PERV). Xenotransplantation. 2000; 7:138–142.
Article14. Specke V, Rubant S, Denner J. Productive infection of human primary cells and cell lines with porcine endogenous retroviruses. Virology. 2001; 285:177–180.
Article15. Wilson CA, Wong S, Muller J, Davidson CE, Rose TM, Burd P. Type C retrovirus released from porcine primary peripheral blood mononuclear cells infects human cells. J Virol. 1998; 72:3082–3087.
Article16. Czauderna F, Fischer N, Boller K, Kurth R, Tönjes RR. Establishment and characterization of molecular clones of porcine endogenous retroviruses replicating on human cells. J Virol. 2000; 74:4028–4038.
Article17. Krach U, Fischer N, Czauderna F, Tönjes RR. Comparison of replication-competent molecular clones of porcine endogenous retrovirus class A and class B derived from pig and human cells. J Virol. 2001; 75:5465–5472.
Article18. Tönjes RR, Czauderna F, Fischer N, Krach U, Boller K, Chardon P, Rogel-Gaillard C, Niebert M, Scheef G, Werner A, Kurth R. Molecularly cloned porcine endogenous retroviruses replicate on human cells. Transplant Proc. 2000; 32:1158–1161.
Article19. Huthoff H, Towers GJ. Restriction of retroviral replication by APOBEC3G/F and TRIM5alpha. Trends Microbiol. 2008; 16:612–619.20. Switzer WM, Michler RE, Shanmugam V, Matthews A, Hussain AI, Wright A, Sandstrom P, Chapman LE, Weber C, Safley S, Denny RR, Navarro A, Evans V, Norin AJ, Kwiatkowski P, Heneine W. Lack of cross-species transmission of porcine endogenous retrovirus infection to nonhuman primate recipients of porcine cells, tissues, or organs. Transplantation. 2001; 71:959–965.21. Kim JH, Choi EY, Jung ES, Kwon Y, Lee DS, Hwang DY, Hwang ES. Characterization of clones of human cell line infected with porcine endogenous retrovirus (PERV) from porcine cell line, PK-15. Infect Chemother. 2009; 41:1–8.
Article22. Yu P, Zhang L, Li SF, Li YP, Cheng JQ, Lu YR, Bu H. Long-term effects on HEK-293 cell line after co-culture with porcine endogenous retrovirus. Transplant Proc. 2005; 37:496–499.
Article23. Yu P, Zhang L, Li SF, Cheng JQ, Lu YR, Zeng YZ, Li YP, Bu H. A rapid method for detection of the copy number of porcine endogenous retrovirus in swine. J Rapid Methods Autom Microbiol. 2007; 15:199–205.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Analysis of env Subtypes of Porcine Endogenous Retrovirus in SNU Miniature Pigs
- Construction of the Porcine Endogenous Retrovirus Envelope Glycoprotein A and B Specific Antibody
- Characterization of Clones of Human Cell Line Infected with Porcine Endogenous Retrovirus (PERV) from Porcine Cell Line, PK-15
- Molecular Cloning of PERV-A and PERV-B Envelope Genes from Miniature Pigs
- No expression of porcine endogenous retrovirus after pig to monkey xenotransplantation