J Vet Sci.
2009 Dec;10(4):317-322. 10.4142/jvs.2009.10.4.317.
Comparison of the age-related porcine endogenous retrovirus (PERV) expression using duplex RT-PCR
- Affiliations
-
- 1Department of Veterinary Medicine Virology Lab, College of Veterinary Medicine and BK21 Program for Veterinary Science, Seoul National University, Seoul 151-742, Korea. parkx026@snu.ac.kr
- KMID: 1726904
- DOI: http://doi.org/10.4142/jvs.2009.10.4.317
Abstract
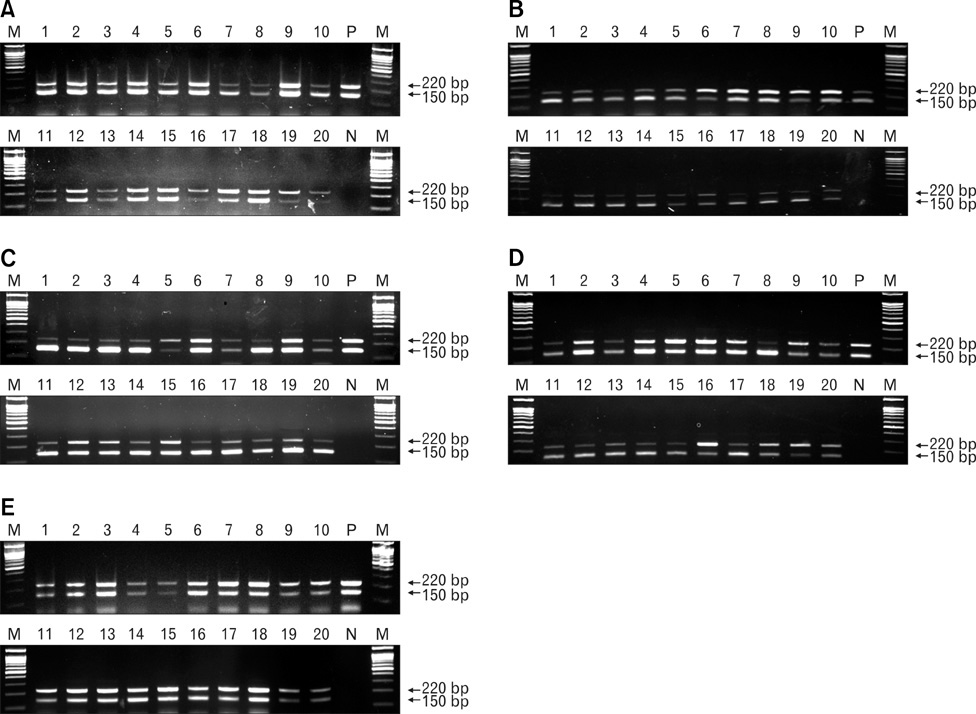
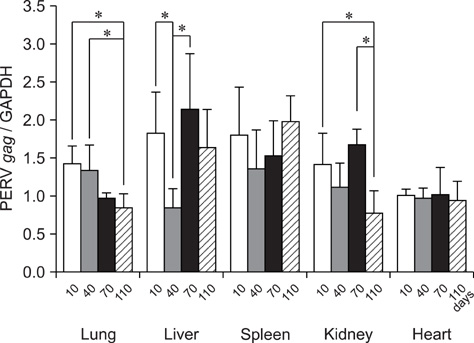
- Porcine endogenous retroviruses (PERVs) are members of family Retroviridae, genus Gamma retrovirus, and transmitted by both horizontally and vertically like other endogenous retroviruses (ERVs). PERV was initially described in the 1970s having inserted its gene in the host genome of different pig breeds, and three classes, PERV-A, PERV-B, and PERV-C are known. The therapeutic use of living cells, tissues, and organs from animals called xenotransplantation might relieve the limited supply of allografts in the treatment of organ dysfunction. Because of ethical considerations, compatible organ sizes, and physiology, the pig has been regarded as an alternative source for xenotransplantation. Sensitive duplex reverse transcription-polymerase chain reaction protocols for simultaneously detecting PERV gag mRNA and porcine glyceraldehydes 3-phosphate dehydrogenase mRNA in one tube was established. To compare the age-related PERV expression patterns of the lung, liver, spleen, kidney, heart, and pancreas in commercial pigs, 20 pigs from four age groups (5 heads each in 10 days-, 40 days-, 70 days-, and 110 days-old, respectively) were used in this study. The expression patterns of PERV were statistically different among age groups in lung, liver, and kidney (ANOVA, p<0.05). These data may support in the selection of appropriate donor pigs expressing low levels of PERV mRNA.
Keyword
MeSH Terms
Figure
Reference
-
1. Akiyoshi DE, Denaro M, Zhu H, Greenstein JL, Banerjee P, Fishman JA. Identification of a full-length cDNA for an endogenous retrovirus of miniature swine. J Virol. 1998. 72:4503–4507.
Article2. Argaw T, Ritzhaupt A, Wilson CA. Development of a real time quantitative PCR assay for detection of porcine endogenous retrovirus. J Virol Methods. 2002. 106:97–106.
Article3. Blusch JH, Roos C, Nitschko H. A polymerase chain reaction-based protocol for the detection of transmission of pig endogenous retroviruses in pig to human xenotransplantation. Transplantation. 2000. 69:2167–2172.
Article4. Breese SS Jr. Virus-like particles occurring in cultures of stable pig kidney cell lines. Arch Gesamte Virusforsch. 1970. 30:401–404.
Article5. Chiang CY, Chang JT, Lin MS, Wang SR, Chang HY. Characterization of a monoclonal antibody specific to the Gag protein of porcine endogenous retrovirus and its application in detecting the virus infection. Virus Res. 2005. 108:139–148.
Article6. Chirgwin JM, Przybyla AE, MacDonald RJ, Rutter WJ. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979. 18:5294–5299.
Article7. Cho K, Lee YK, Greenhalgh DG. Endogenous retroviruses in systemic response to stress signals. Shock. 2008. 30:105–116.
Article8. Clémenceau B, Lalain S, Martignat L, Saï P. Porcine endogenous retroviral mRNAs in pancreas and a panel of tissues from specific pathogen-free pigs. Diabetes Metab. 1999. 25:518–525.9. Czauderna F, Fischer N, Boller K, Kurth R, Tönjes RR. Establishment and characterization of molecular clones of porcine endogenous retroviruses replicating on human cells. J Virol. 2000. 74:4028–4038.
Article10. Fischer N, Krach U, Niebert M, Tönjes RR. Detection of porcine endogenous retrovirus (PERV) using highly specific antisera against Gag and Env. Virology. 2003. 311:222–228.
Article11. Heneine W, Tibell A, Switzer WM, Sandstrom P, Rosales GV, Mathews A, Korsgren O, Chapman LE, Folks TM, Groth CG. No evidence of infection with porcine endogenous retrovirus in recipients of porcine islet-cell xenografts. Lancet. 1998. 352:695–699.
Article12. Jin H, Inoshima Y, Wu D, Morooka A, Sentsui H. Expression of porcine endogenous retrovirus in peripheral blood leukocytes from ten different breeds. Transpl Infect Dis. 2000. 2:11–14.
Article13. Kowalski PE, Freeman JD, Mager DL. Intergenic splicing between a HERV-H endogenous retrovirus and two adjacent human genes. Genomics. 1999. 57:371–379.
Article14. Lee YK, Chew A, Phan H, Greenhalgh DG, Cho K. Genome-wide expression profiles of endogenous retroviruses in lymphoid tissues and their biological properties. Virology. 2008. 373:263–273.
Article15. Löwer R, Löwer J, Kurth R. The viruses in all of us: characteristics and biological significance of human endogenous retrovirus sequences. Proc Natl Acad Sci USA. 1996. 93:5177–5184.
Article16. Moon HJ, Park SJ, Yang JS, Lee CS, Song DS, Kang BK, Park BK. Establishment of PCR and RT-PCR for porcine endogenous retrovirus detection. Korean J Vet Public Health. 2008. 32:265–271.17. Patience C, Switzer WM, Takeuchi Y, Griffiths DJ, Goward ME, Heneine W, Stoye JP, Weiss RA. Multiple groups of novel retroviral genomes in pigs and related species. J Virol. 2001. 75:2771–2775.
Article18. Quinn G, Wood J, Suling K, Arn S, Sachs DH, Schuurman HJ, Patience C. Genotyping of porcine endogenous retroviruses from a family of miniature swine. J Virol. 2004. 78:314–319.
Article19. Swindle MM. Defining appropriate health status and management programs for specific-pathogen-free swine for xenotransplantation. Ann N Y Acad Sci. 1998. 862:111–120.
Article20. Switzer WM, Shanmugam V, Chapman L, Heneine W. Polymerase chain reaction assays for the diagnosis of infection with the porcine endogenous retrovirus and the detection of pig cells in human and nonhuman recipients of pig xenografts. Transplantation. 1999. 68:183–188.
Article21. Takeuchi Y, Patience C, Magre S, Weiss RA, Banerjee PT, Le Tissier P, Stoye JP. Host range and interference studies of three classes of pig endogenous retrovirus. J Virol. 1998. 72:9986–9991.
Article22. Taruscio D, Mantovani A. Factors regulating endogenous retroviral sequences in human and mouse. Cytogenet Genome Res. 2004. 105:351–362.
Article23. Tucker AW, Mellencamp MM, Donadeu M, Scobie L. Retroviremia in commercial pigs and its preliminary association with poor health. J Clin Microbiol. 2006. 44:3846–3847.
Article24. Wood JC, Quinn G, Suling KM, Oldmixon BA, Van Tine BA, Cina R, Arn S, Huang CA, Scobie L, Onions DE, Sachs DH, Schuurman HJ, Fishman JA, Patience C. Identification of exogenous forms of human-tropic porcine endogenous retrovirus in miniature Swine. J Virol. 2004. 78:2494–2501.
Article25. Yu P, Zhang L, Li SF, Cheng JQ, Lu YR, Li YP, Bu H. Transmission of porcine endogenous retrovirus to human cells in nude mouse. Acta Virol. 2008. 52:257–260.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Analysis of env Subtypes of Porcine Endogenous Retrovirus in SNU Miniature Pigs
- Detection of Human Cytomegalovirus (HCMV) and Porcine Endogenous Retrovirus (PERV) with One Step Extraction Method
- Construction of the Porcine Endogenous Retrovirus Envelope Glycoprotein A and B Specific Antibody
- Characterization of Clones of Human Cell Line Infected with Porcine Endogenous Retrovirus (PERV) from Porcine Cell Line, PK-15
- No expression of porcine endogenous retrovirus after pig to monkey xenotransplantation