Int J Stem Cells.
2021 Nov;14(4):447-454. 10.15283/ijsc21050.
Hypoxic Pretreatment of Adipose-Derived Stem Cells Accelerates Diabetic Wound Healing via circ-Gcap14 and HIF-1α/VEGF Mediated Angiopoiesis
- Affiliations
-
- 1Department of Plastic & Cosmetic Surgery, Peking Union Medical College Hospital, Beijing, China
- KMID: 2522566
- DOI: http://doi.org/10.15283/ijsc21050
Abstract
- Background and Objectives
Adipose-derived stem cell (ADSC) transplantation improves stem cell paracrine function and can enhance wound healing. However, in diabetic patients, glucose-associated effects on this function and cell survival lead to impaired wound closure, thereby limiting ADSC transplantation efficiency. The hypoxia-inducible factor HIF-1α has an important protective function during wound healing. Here, we aim to clarify the regulatory mechanism of ADSCs.
Methods and Results
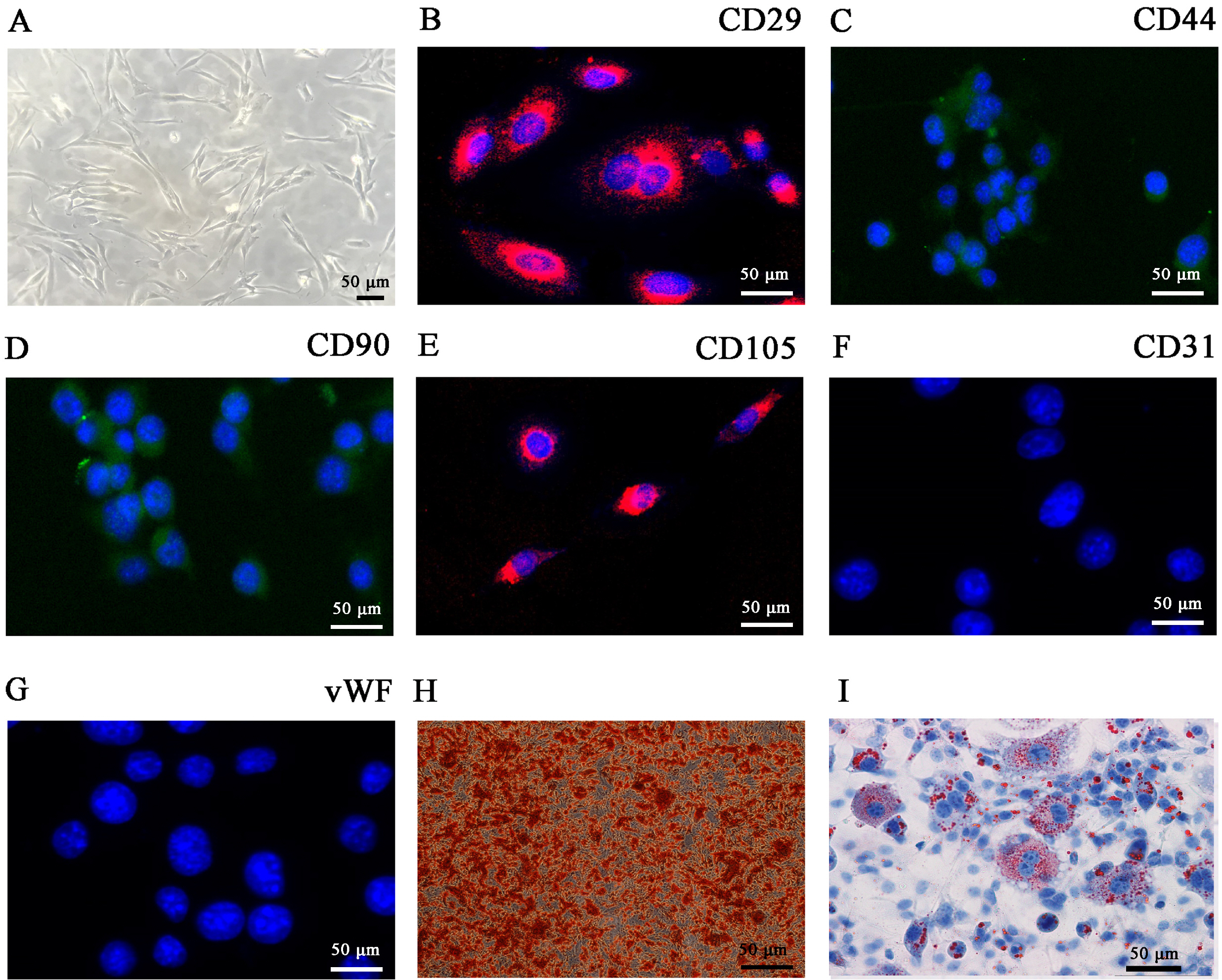
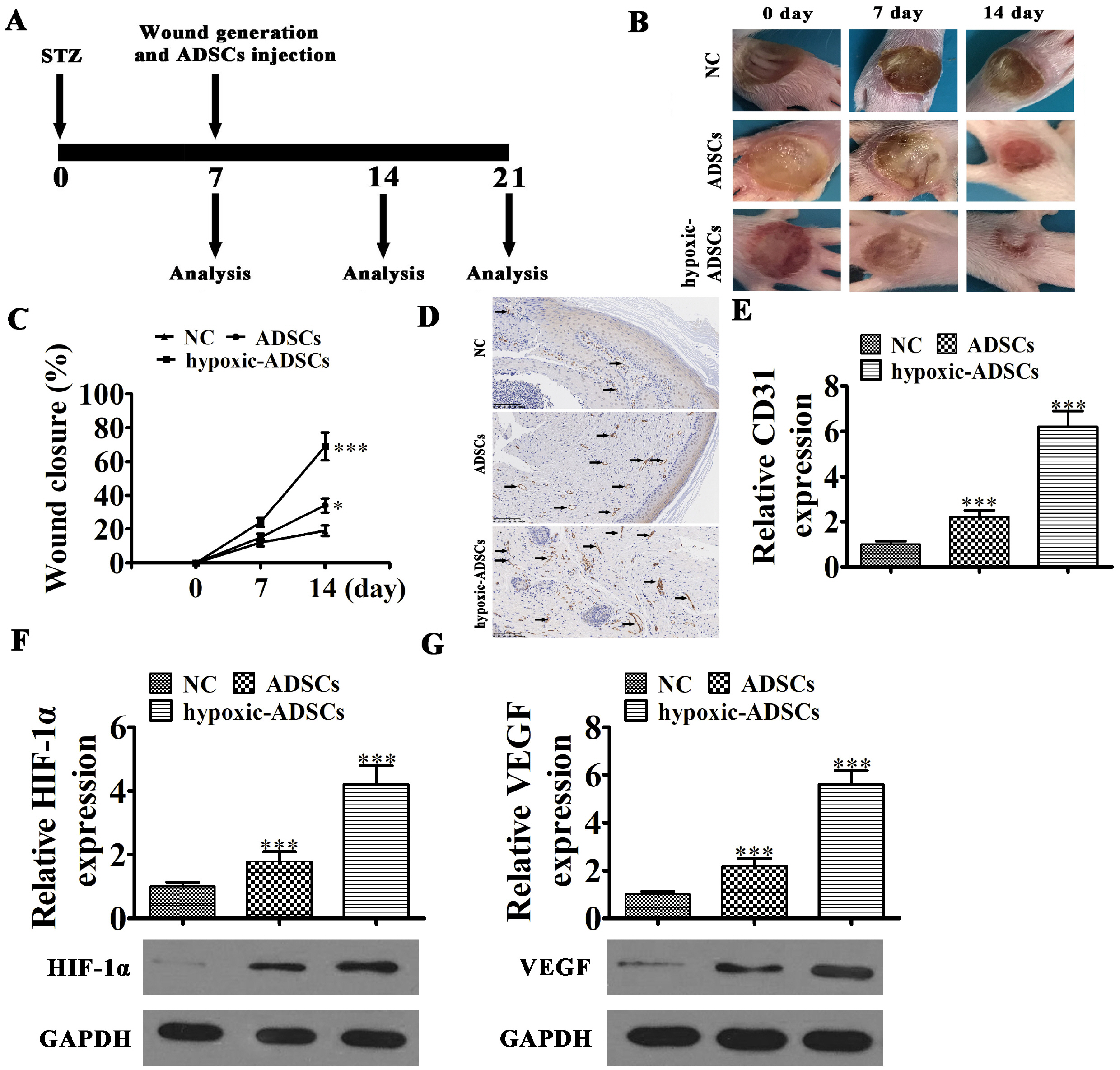
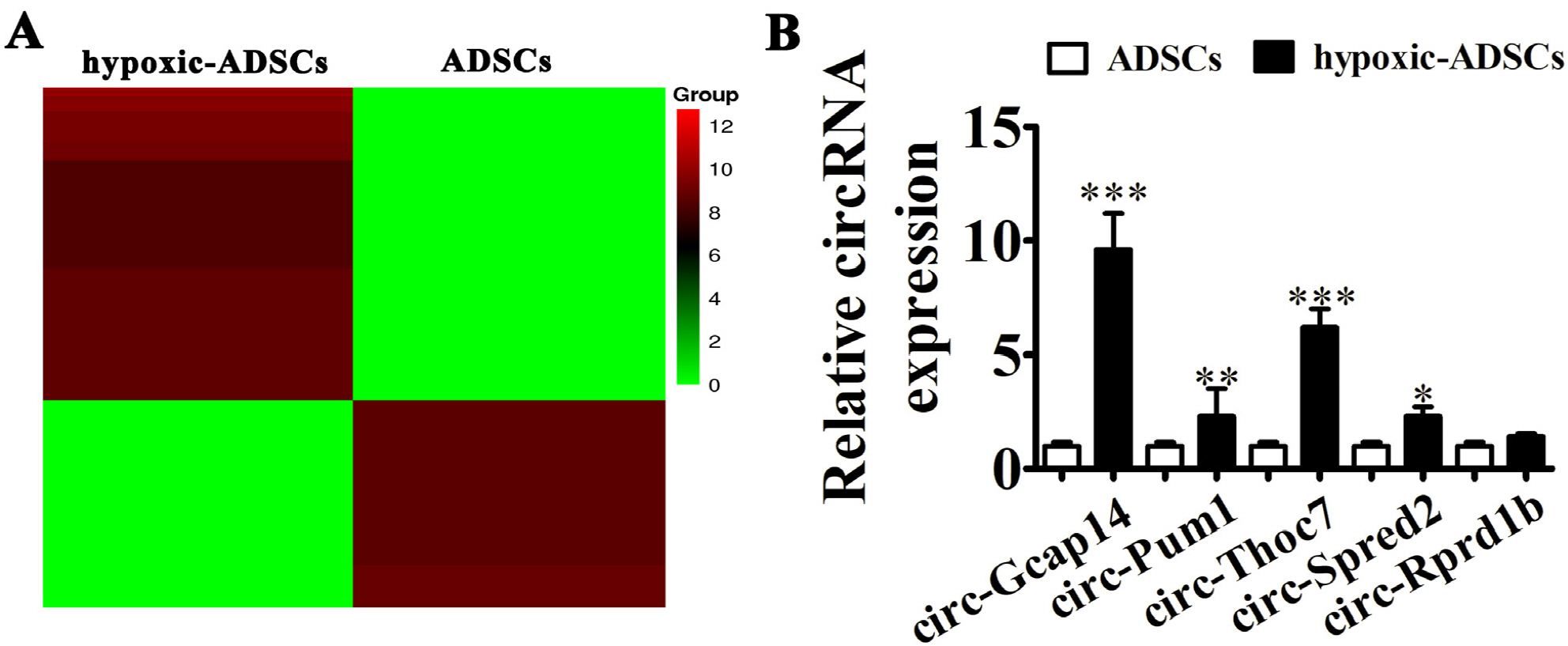
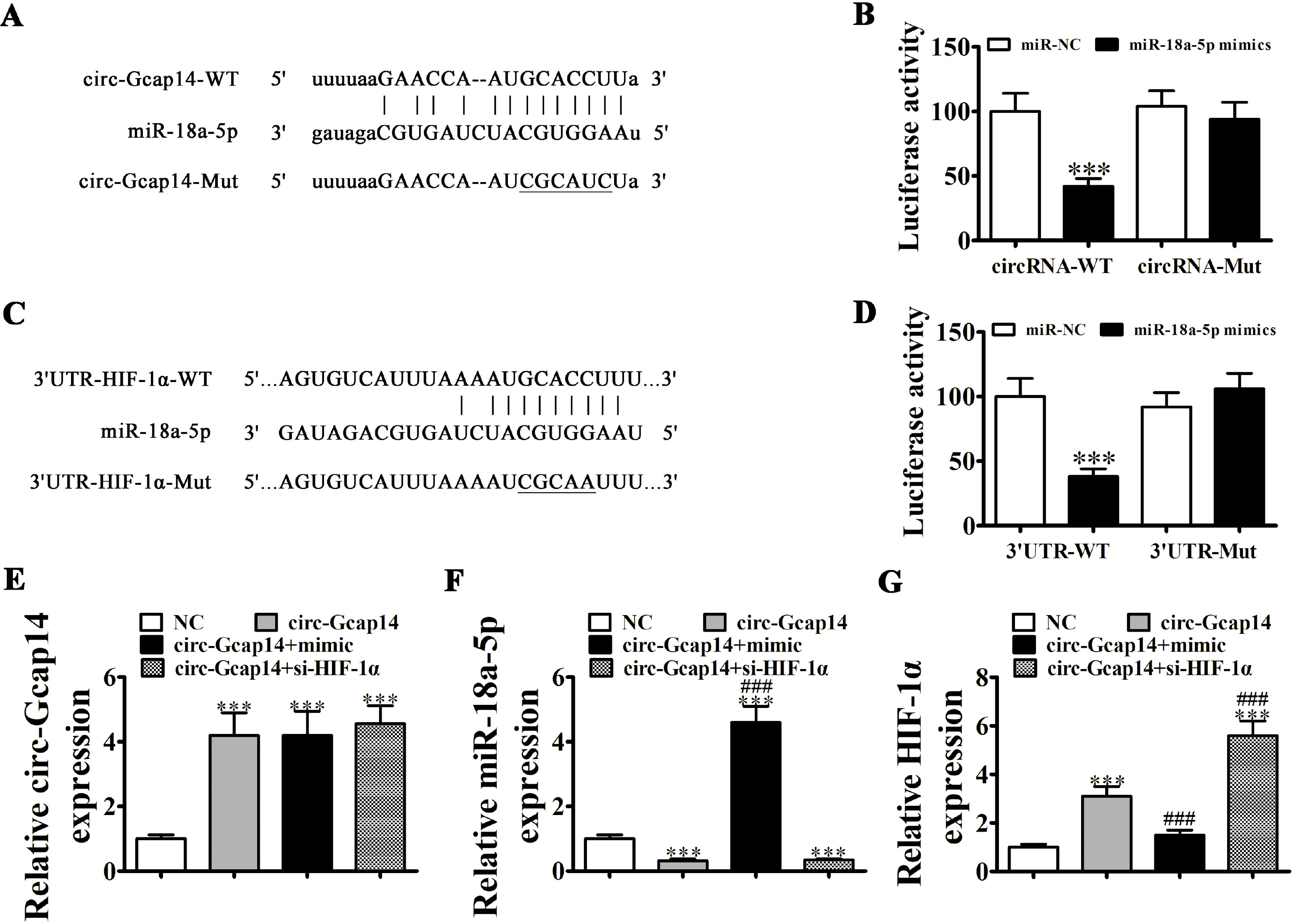
ADSCs were isolated from BALB/C mice adipose samples. We then used high-throughput se-quencing to assess abnormal expression of circular RNAs (circRNAs). We also used an in vivo full-thickness skin defect mouse model to assess the effects of transplanted ADSC on diabetic wound closure. Hypoxic pretreatment of ADSCs accelerated diabetic wound closure, which enhanced angiogenic growth factor expression in our mouse model. High-throughput sequencing and RT-qPCR indicated that circ-Gcap14 was upregulated in hypoxic pretreated ADSCs. Similarly, circ-Gcap14 downregulation also decreased the therapeutic effects of ADSCs; however, circ-Gcap14 overexpression increased the effects of ADSC by promoting angiopoiesis. We also used a luciferase reporter assay to confirm that miR-18a-5p and HIF-1α were downstream targets of circ-Gcap14. HIF-1α expression plays an important role in increased VEGF level.
Conclusions
Based on our data, we suggest that circ-Gcap14 plays an important role in accelerating hypoxic ADSC-mediated diabetic wound closure, by enhancing mouse angiogenic growth factor expression and regulating downstream miR-18a-5p/HIF-1α expression.
Figure
Reference
-
References
1. Haddad JA, Haddad AN. 2018; The past decade in type 2 diabetes and future challenges. Hormones (Athens). 17:451–459. DOI: 10.1007/s42000-018-0080-y. PMID: 30519831.
Article2. Davis FM, Kimball A, Boniakowski A, Gallagher K. 2018; Dysfunctional wound healing in diabetic foot ulcers: new crossroads. Curr Diab Rep. 18:2. DOI: 10.1007/s11892-018-0970-z. PMID: 29362914.
Article3. Tao SC, Rui BY, Wang QY, Zhou D, Zhang Y, Guo SC. 2018; Extracellular vesicle-mimetic nanovesicles transport LncRNA-H19 as competing endogenous RNA for the treatment of diabetic wounds. Drug Deliv. 25:241–255. DOI: 10.1080/10717544.2018.1425774. PMID: 29334272. PMCID: PMC6058500.
Article4. Chalmers J, Joshi R, Patel A. 2008; Advances in reducing the burden of vascular disease in type 2 diabetes. Clin Exp Pharmacol Physiol. 35:434–437. DOI: 10.1111/j.1440-1681.2008.04892.x. PMID: 18307736.
Article5. Xu J, Liu X, Zhao F, Zhang Y, Wang Z. 2020; HIF1α overexpression enhances diabetic wound closure in high glucose and low oxygen conditions by promoting adipose-derived stem cell paracrine function and survival. Stem Cell Res Ther. 11:148. DOI: 10.1186/s13287-020-01654-2. PMID: 32248837. PMCID: PMC7132964.
Article6. Wang Z, Li H, Zhang D, Liu X, Zhao F, Pang X, Wang Q. 2015; Effect of advanced glycosylation end products on apoptosis in human adipose tissue-derived stem cells in vitro. Cell Biosci. 5:3. DOI: 10.1186/2045-3701-5-3. PMID: 25973170. PMCID: PMC4429817.
Article7. Hwang OK, Noh YW, Hong JT, Lee JW. 2020; Hypoxia pretreatment promotes chondrocyte differentiation of human adipose-derived stem cells via vascular endothelial growth factor. Tissue Eng Regen Med. 17:335–350. DOI: 10.1007/s13770-020-00265-5. PMID: 32451775. PMCID: PMC7260353.
Article8. Wu H, Wu S, Zhu Y, Ye M, Shen J, Liu Y, Zhang Y, Bu S. 2019; Hsa_circRNA_0054633 is highly expressed in gestational diabetes mellitus and closely related to glycosylation index. Clin Epigenetics. 11:22. DOI: 10.1186/s13148-019-0610-8. PMID: 30736847. PMCID: PMC6368772.
Article9. Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer M, Loewer A, Ziebold U, Landthaler M, Kocks C, le Noble F, Rajewsky N. 2013; Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 495:333–338. DOI: 10.1038/nature11928. PMID: 23446348.
Article10. Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, Kjems J. 2013; Natural RNA circles function as efficient microRNA sponges. Nature. 495:384–388. DOI: 10.1038/nature11993. PMID: 23446346.
Article11. Fischer JW, Leung AK. 2017; CircRNAs: a regulator of cellular stress. Crit Rev Biochem Mol Biol. 52:220–233. DOI: 10.1080/10409238.2016.1276882. PMID: 28095716. PMCID: PMC5526226.
Article12. Geng W, Tang H, Luo S, Lv Y, Liang D, Kang X, Hong W. 2019; Exosomes from miRNA-126-modified ADSCs promotes functional recovery after stroke in rats by improving neurogenesis and suppressing microglia activation. Am J Transl Res. 11:780–792. PMID: 30899379. PMCID: PMC6413259.13. Zhang AJ, Jiang T, Li Q, Jin PS, Tan Q. 2018; Experimental research on ADSCs-NCSS in wound repair. Exp Ther Med. 16:4429–4436. DOI: 10.3892/etm.2018.6756. PMID: 30542393. PMCID: PMC6257557.
Article14. Qu Y, Zhang Q, Cai X, Li F, Ma Z, Xu M, Lu L. 2017; Exosomes derived from miR-181-5p-modified adipose-derived mesenchymal stem cells prevent liver fibrosis via autophagy activation. J Cell Mol Med. 21:2491–2502. DOI: 10.1111/jcmm.13170. PMID: 28382720. PMCID: PMC5618698.
Article15. Li Q, Xia S, Yin Y, Guo Y, Chen F, Jin P. 2018; miR-5591-5p regulates the effect of ADSCs in repairing diabetic wound via targeting AGEs/AGER/JNK signaling axis. Cell Death Dis. 9:566. DOI: 10.1038/s41419-018-0615-9. PMID: 29752466. PMCID: PMC5948214.
Article16. Fernando EH, Gordon MH, Beck PL, MacNaughton WK. 2018; Inhibition of intestinal epithelial wound healing through protease-activated receptor-2 activation in Caco2 cells. J Pharmacol Exp Ther. 367:382–392. DOI: 10.1124/jpet.118.249524. PMID: 30190338.
Article17. Zhi Z, Yang W, Liu L, Jiang X, Pang L. 2018; Early missed abortion is associated with villous angiogenesis via the HIF-1α/VEGF signaling pathway. Arch Gynecol Obstet. 298:537–543. DOI: 10.1007/s00404-018-4802-9. PMID: 29951709. PMCID: PMC6096576.
Article18. Lin CJ, Lan YM, Ou MQ, Ji LQ, Lin SD. 2019; Expression of miR-217 and HIF-1α/VEGF pathway in patients with diabetic foot ulcer and its effect on angiogenesis of diabetic foot ulcer rats. J Endocrinol Invest. 42:1307–1317. DOI: 10.1007/s40618-019-01053-2. PMID: 31079353.
Article19. Falanga V. 2005; Wound healing and its impairment in the diabetic foot. Lancet. 366:1736–1743. DOI: 10.1016/S0140-6736(05)67700-8. PMID: 16291068.
Article20. Zhang J, Chen C, Hu B, Niu X, Liu X, Zhang G, Zhang C, Li Q, Wang Y. 2016; Exosomes derived from human endothelial progenitor cells accelerate cutaneous wound healing by promoting angiogenesis through Erk1/2 signaling. Int J Biol Sci. 12:1472–1487. DOI: 10.7150/ijbs.15514. PMID: 27994512. PMCID: PMC5166489.
Article21. Li C, Wang Q, Shen S, Wei X, Li G. 2019; HIF-1α/VEGF signaling-mediated epithelial-mesenchymal transition and angiogenesis is critically involved in anti-metastasis effect of luteolin in melanoma cells. Phytother Res. 33:798–807. DOI: 10.1002/ptr.6273. PMID: 30653763. PMCID: PMC6590488.
Article22. Yu Z, Zhang T, Gong C, Sheng Y, Lu B, Zhou L, Ji L, Wang Z. 2016; Erianin inhibits high glucose-induced retinal angiogenesis via blocking ERK1/2-regulated HIF-1α-VEGF/VEGFR2 signaling pathway. Sci Rep. 6:34306. DOI: 10.1038/srep34306. PMID: 27678303. PMCID: PMC5039671.
Article23. Nan Y, Guo H, Guo L, Wang L, Ren B, Yu K, Huang Q, Zhong Y. 2018; MiRNA-451 inhibits glioma cell proliferation and invasion through the mTOR/HIF-1α/VEGF signaling pathway by targeting CAB39. Hum Gene Ther Clin Dev. 29:156–166. DOI: 10.1089/humc.2018.133. PMID: 30180756.
Article24. Yu WY, Sun W, Yu DJ, Zhao TL, Wu LJ, Zhuang HR. 2018; Adipose-derived stem cells improve neovascularization in ischemic flaps in diabetic mellitus through HIF-1α/VEGF pathway. Eur Rev Med Pharmacol Sci. 22:10–16. DOI: 10.26355/eurrev_201801_14094. PMID: 29364466.25. Wu J, Ke X, Wang W, Zhang H, Ma N, Fu W, Zhao M, Gao X, Hao X, Zhang Z. 2016; Aloe-emodin suppresses hypoxia-induced retinal angiogenesis via inhibition of HIF-1α/VEGF pathway. Int J Biol Sci. 12:1363–1371. DOI: 10.7150/ijbs.16334. PMID: 27877088. PMCID: PMC5118782.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Extracellular Vesicles from Adipose-Derived Stem Cells Promote Diabetic Wound Healing via the PI3K-AKT-mTOR-HIF-1a Signaling Pathway
- Role of HIF-1α in the Responses of Tumors to Radiotherapy and Chemotherapy
- Proliferation of Keratinocytes Induced by Adipose-Derived Stem Cells on a Chitosan Scaffold and Its Role in Wound Healing, a Review
- Hypoxia-Inducible Factor-1 Alpha Stabilization in Human Macrophages during Leishmania major Infection Is Impaired by Parasite Virulence
- Effects of Human Adipose-derived Stem Cells on Cutaneous Wound Healing in Nude Mice