Cancer Res Treat.
2025 Jan;57(1):1-10. 10.4143/crt.2024.255.
Role of HIF-1α in the Responses of Tumors to Radiotherapy and Chemotherapy
- Affiliations
-
- 1Department of Radiation Oncology, University of Minnesota Medical School, Minneapolis, MN, USA
- 2Department of Radiation Oncology, Korea Institute of Radiological & Medical Sciences, Seoul, Korea
- 3Department of Microbiology, College of Medicine, Inha University, Incheon, Korea
- 4Department of Neurosurgery, Seoul National University College of Medicine, Seoul, Korea
- KMID: 2564575
- DOI: http://doi.org/10.4143/crt.2024.255
Abstract
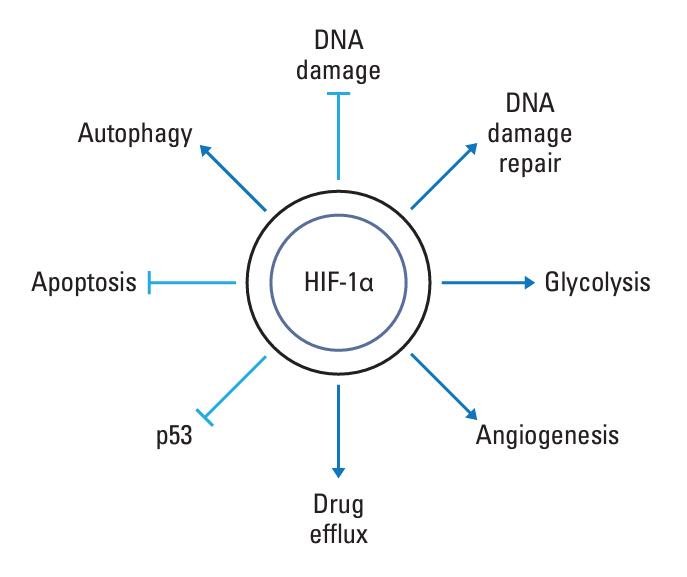
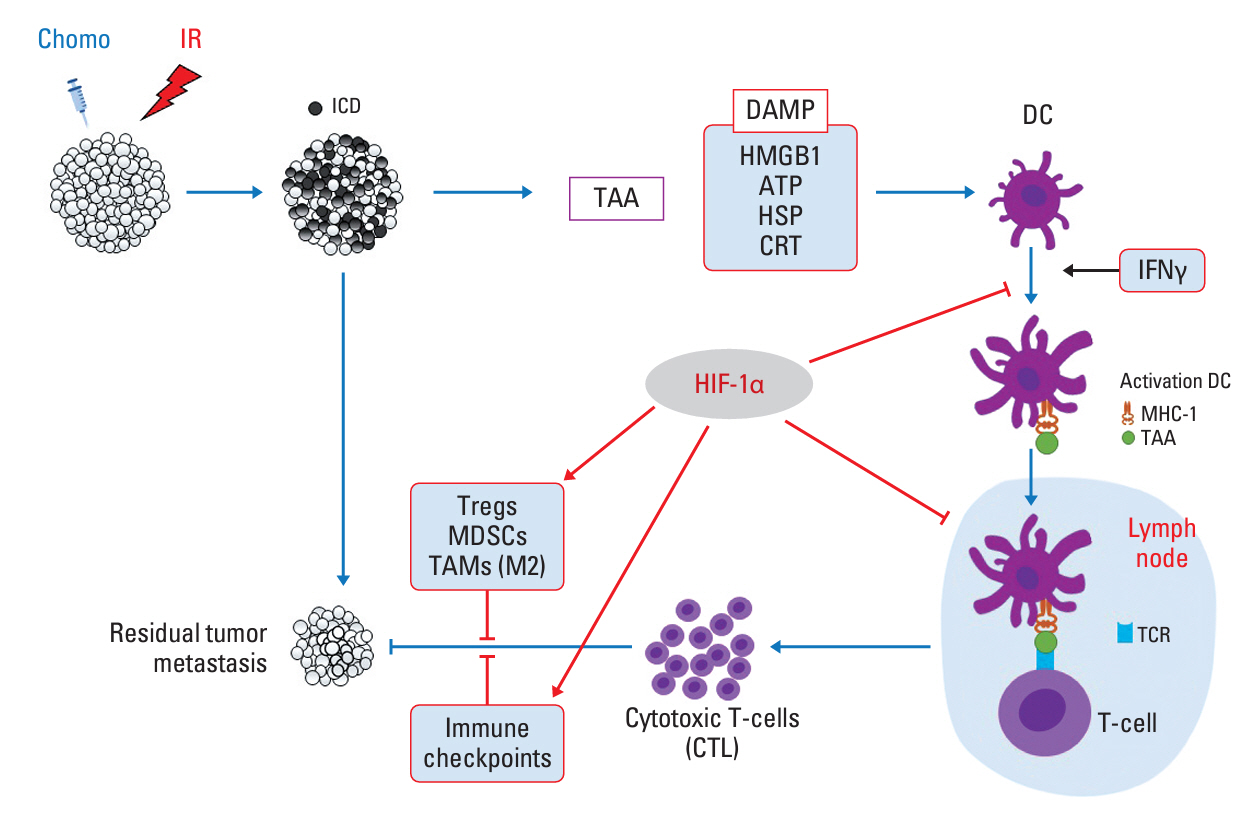
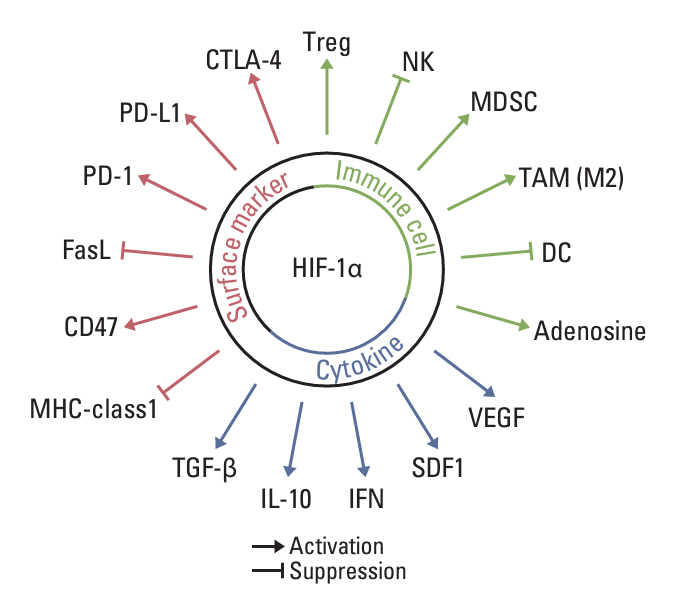
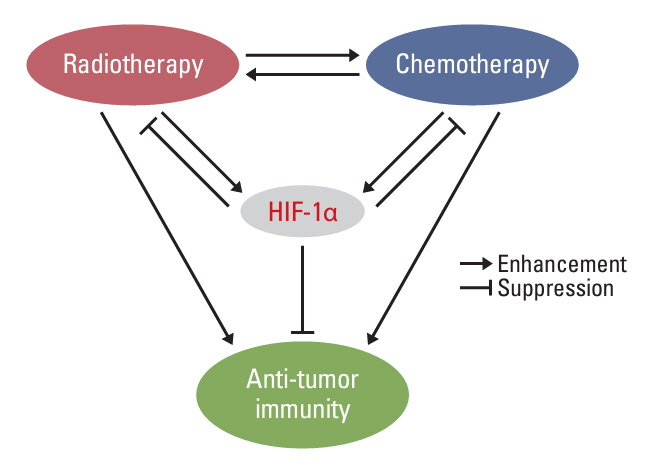
- Tumor microenvironment is intrinsically hypoxic with abundant hypoxia-inducible factors-1α (HIF-1α), a primary regulator of the cellular response to hypoxia and various stresses imposed on the tumor cells. HIF-1α increases radioresistance and chemoresistance by reducing DNA damage, increasing repair of DNA damage, enhancing glycolysis that increases antioxidant capacity of tumors cells, and promoting angiogenesis. In addition, HIF-1α markedly enhances drug efflux, leading to multidrug resistance. Radiotherapy and certain chemotherapy drugs evoke profound anti-tumor immunity by inducing immunologic cell death that release tumor-associated antigens together with numerous pro-immunological factors, leading to priming of cytotoxic CD8+ T cells and enhancing the cytotoxicity of macrophages and natural killer cells. Radiotherapy and chemotherapy of tumors significantly increase HIF-1α activity in tumor cells. Unfortunately, HIF-1α effectively promotes various immune suppressive pathways including secretion of immune suppressive cytokines, activation of myeloid-derived suppressor cells, activation of regulatory T cells, inhibition of T cells priming and activity, and upregulation of immune checkpoints. Consequently, the anti-tumor immunity elevated by radiotherapy and chemotherapy is counterbalanced or masked by the potent immune suppression promoted by HIF-1α. Effective inhibition of HIF-1α may significantly increase the efficacy of radiotherapy and chemotherapy by increasing radiosensitivity and chemosensitivity of tumor cells and also by upregulating anti-tumor immunity.
Keyword
Figure
Reference
-
References
1. Grimm J, Marks LB, Jackson A, Kavanagh BD, Xue J, Yorke E. High dose per fraction, hypofractionated treatment effects in the clinic (HyTEC): an overview. Int J Radiat Oncol Biol Phys. 2021; 110:1–10.
Article2. Song CW, Glatstein E, Marks LB, Emami B, Grimm J, Sperduto PW, et al. Biological principles of stereotactic body radiation therapy (SBRT) and stereotactic radiation surgery (SRS): indirect cell death. Int J Radiat Oncol Biol Phys. 2021; 110:21–34.
Article3. Malouff TD, Mahajan A, Krishnan S, Beltran C, Seneviratne DS, Trifiletti DM. Carbon ion therapy: a modern review of an emerging technology. Front Oncol. 2020; 10:82.
Article4. Song CW, Terezakis S, Park WY, Paek SH, Kim MS, Cho LC, et al. Preferential tumor vascular damage is the common antitumor mechanism of high-dose hypofractionated radiation therapy: SABR, spatially fractionated radiation therapy, and FLASH radiation therapy. Int J Radiat Oncol Biol Phys. 2023; 117:701–4.
Article5. Bertsimas D, O’Hair A, Relyea S, Silberholz J. An analytics approach to designing combination chemotherapy regimens for cancer. Manage Sci. 2016; 62:1511–31.
Article6. Debela DT, Muzazu SG, Heraro KD, Ndalama MT, Mesele BW, Haile DC, et al. New approaches and procedures for cancer treatment: current perspectives. SAGE Open Med. 2021; 9:20503121211034366.
Article7. Nadiradze G, Horvath P, Sautkin Y, Archid R, Weinreich FJ, Konigsrainer A, et al. Overcoming drug resistance by taking advantage of physical principles: pressurized intraperitoneal aerosol chemotherapy (PIPAC). Cancers (Basel). 2019; 12:34.
Article8. Vobugari N, Raja V, Sethi U, Gandhi K, Raja K, Surani SR. Advancements in oncology with artificial intelligence: a review article. Cancers (Basel). 2022; 14:1349.9. Schwartz DL, Bankson J, Bidaut L, He Y, Williams R, Lemos R, et al. HIF-1-dependent stromal adaptation to ischemia mediates in vivo tumor radiation resistance. Mol Cancer Res. 2011; 9:259–70.10. Huang R, Zhou PK. HIF-1 signaling: a key orchestrator of cancer radioresistance. Radiat Med Prot. 2020; 1:7–14.
Article11. Moeller BJ, Dewhirst MW. HIF-1 and tumour radiosensitivity. Br J Cancer. 2006; 95:1–5.
Article12. Moeller BJ, Dreher MR, Rabbani ZN, Schroeder T, Cao Y, Li CY, et al. Pleiotropic effects of HIF-1 blockade on tumor radiosensitivity. Cancer Cell. 2005; 8:99–110.
Article13. Xia Y, Jiang L, Zhong T. The role of HIF-1alpha in chemo-/radioresistant tumors. Onco Targets Ther. 2018; 11:3003–11.14. Harada H. Hypoxia-inducible factor 1-mediated characteristic features of cancer cells for tumor radioresistance. J Radiat Res. 2016; 57 Suppl 1:i99–105.
Article15. Wennerberg E, Lhuillier C, Vanpouille-Box C, Pilones KA, Garcia-Martinez E, Rudqvist NP, et al. Barriers to radiation-induced in situ tumor vaccination. Front Immunol. 2017; 8:229.
Article16. Pinzon-Daza ML, Cuellar-Saenz Y, Nualart F, Ondo-Mendez A, Del Riesgo L, Castillo-Rivera F, et al. Oxidative stress promotes doxorubicin-induced Pgp and BCRP expression in colon cancer cells under hypoxic conditions. J Cell Biochem. 2017; 118:1868–78.
Article17. Tang YA, Chen YF, Bao Y, Mahara S, Yatim S, Oguz G, et al. Hypoxic tumor microenvironment activates GLI2 via HIF1-alpha and TGF-beta2 to promote chemoresistance in colorectal cancer. Proc Natl Acad Sci U S A. 2018; 115:E5990–9.18. Okazaki M, Fushida S, Tsukada T, Kinoshita J, Oyama K, Miyashita T, et al. The effect of HIF-1alpha and PKM1 expression on acquisition of chemoresistance. Cancer Manag Res. 2018; 10:1865–74.19. Zhao Q, Li Y, Tan BB, Fan LQ, Yang PG, Tian Y. HIF-1alpha induces multidrug resistance in gastric cancer cells by inducing MiR-27a. PLoS One. 2015; 10:e0132746.20. Gao XZ, Wang GN, Zhao WG, Han J, Diao CY, Wang XH, et al. Blocking OLFM4/HIF-1alpha axis alleviates hypoxia-induced invasion, epithelial-mesenchymal transition, and chemotherapy resistance in non-small-cell lung cancer. J Cell Physiol. 2019; 234:15035–43.21. Wu HM, Jiang ZF, Ding PS, Shao LJ, Liu RY. Hypoxia-induced autophagy mediates cisplatin resistance in lung cancer cells. Sci Rep. 2015; 5:12291.
Article22. Wen YA, Stevens PD, Gasser ML, Andrei R, Gao T. Downregulation of PHLPP expression contributes to hypoxia-induced resistance to chemotherapy in colon cancer cells. Mol Cell Biol. 2013; 33:4594–605.
Article23. Brown LM, Cowen RL, Debray C, Eustace A, Erler JT, Sheppard FC, et al. Reversing hypoxic cell chemoresistance in vitro using genetic and small molecule approaches targeting hypoxia inducible factor-1. Mol Pharmacol. 2006; 69:411–8.
Article24. Hussein D, Estlin EJ, Dive C, Makin GW. Chronic hypoxia promotes hypoxia-inducible factor-1alpha-dependent resistance to etoposide and vincristine in neuroblastoma cells. Mol Cancer Ther. 2006; 5:2241–50.25. Deben C, Deschoolmeester V, De Waele J, Jacobs J, Van den Bossche J, Wouters A, et al. Hypoxia-induced cisplatin resistance in non-small cell lung cancer cells is mediated by HIF1-alpha and mutant p53 and can be overcome by induction of oxidative stress. Cancers (Basel). 2018; 10:126.
Article26. Warfel NA, El-Deiry WS. HIF-1 signaling in drug resistance to chemotherapy. Curr Med Chem. 2014; 21:3021–8.
Article27. Lv Y, Zhao S, Han J, Zheng L, Yang Z, Zhao L. Hypoxia-inducible factor-1alpha induces multidrug resistance protein in colon cancer. Onco Targets Ther. 2015; 8:1941–8.28. Rohwer N, Cramer T. Hypoxia-mediated drug resistance: novel insights on the functional interaction of HIFs and cell death pathways. Drug Resist Updat. 2011; 14:191–201.
Article29. Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 2010; 29:625–34.
Article30. Xu K, Zhan Y, Yuan Z, Qiu Y, Wang H, Fan G, et al. Hypoxia induces drug resistance in colorectal cancer through the HIF-1alpha/miR-338-5p/IL-6 feedback loop. Mol Ther. 2019; 27:1810–24.31. Chen J, Ding Z, Peng Y, Pan F, Li J, Zou L, et al. HIF-1alpha inhibition reverses multidrug resistance in colon cancer cells via downregulation of MDR1/P-glycoprotein. PLoS One. 2014; 9:e98882.32. Bergerud KM, Berkseth M, Pardoll DM, Ganguly S, Kleinberg LR, Lawrence J, et al. Radiation therapy and myeloid-derived suppressor cells: breaking down their cancerous partnership. Int J Radiat Oncol Biol Phys. 2024; 119:42–55.
Article33. Demaria S, Formenti SC. Radiation as an immunological adjuvant: current evidence on dose and fractionation. Front Oncol. 2012; 2:153.
Article34. Rapoport BL, Anderson R. Realizing the clinical potential of immunogenic cell death in cancer chemotherapy and radiotherapy. Int J Mol Sci. 2019; 20:959.
Article35. Wang Q, Ju X, Wang J, Fan Y, Ren M, Zhang H. Immunogenic cell death in anticancer chemotherapy and its impact on clinical studies. Cancer Lett. 2018; 438:17–23.
Article36. Wu Q, You L, Nepovimova E, Heger Z, Wu W, Kuca K, et al. Hypoxia-inducible factors: master regulators of hypoxic tumor immune escape. J Hematol Oncol. 2022; 15:77.
Article37. You L, Wu W, Wang X, Fang L, Adam V, Nepovimova E, et al. The role of hypoxia-inducible factor 1 in tumor immune evasion. Med Res Rev. 2021; 41:1622–43.
Article38. Chen Y, Gaber T. Hypoxia/HIF modulates immune responses. Biomedicines. 2021; 9:260.
Article39. Savage T, Guha C. Radio-immunology of ablative radiation. In : Trifiletti D, Chao S, Sahgal A, Sheehan J, editors. Stereotactic radiosurgery and stereotactic body radiation therapy. Cham: Springer;2019. p. 15–29.40. Vito A, El-Sayes N, Mossman K. Hypoxia-driven immune escape in the tumor microenvironment. Cells. 2020; 9:992.
Article41. Song CW, Kim H, Cho H, Kim MS, Paek SH, Park HJ, et al. HIF-1alpha inhibition improves anti-tumor immunity and promotes the efficacy of stereotactic ablative radiotherapy (SABR). Cancers (Basel). 2022; 14:3273.
Article42. Meijer TW, Kaanders JH, Span PN, Bussink J. Targeting hypoxia, HIF-1, and tumor glucose metabolism to improve radiotherapy efficacy. Clin Cancer Res. 2012; 18:5585–94.
Article43. Zaidi N, Jaffee EM. Immunotherapy transforms cancer treatment. J Clin Invest. 2019; 129:46–7.
Article44. Dede Z, Tumer K, Kan T, Yucel B. Current advances and future prospects in cancer immunotherapeutics. Medeni Med J. 2023; 38:88–94.
Article45. Marciscano AE, Haimovitz-Friedman A, Lee P, Tran PT, Tome WA, Guha C, et al. Immunomodulatory effects of stereotactic body radiation therapy: preclinical insights and clinical opportunities. Int J Radiat Oncol Biol Phys. 2021; 110:35–52.
Article46. Jiang W, Tang C, Chang JY. Radiation with immunotherapy: an emerging combination for cancer treatment. J Radiat Oncol. 2015; 4:331–8.
Article47. Zhang Z, Liu X, Chen D, Yu J. Radiotherapy combined with immunotherapy: the dawn of cancer treatment. Signal Transduct Target Ther. 2022; 7:258.
Article48. Fabian KP, Wolfson B, Hodge JW. From immunogenic cell death to immunogenic modulation: select chemotherapy regimens induce a spectrum of immune-enhancing activities in the tumor microenvironment. Front Oncol. 2021; 11:728018.
Article49. Infantino V, Santarsiero A, Convertini P, Todisco S, Iacobazzi V. Cancer cell metabolism in hypoxia: role of HIF-1 as key regulator and therapeutic target. Int J Mol Sci. 2021; 22:5703.
Article50. Gao P, Niu N, Wei T, Tozawa H, Chen X, Zhang C, et al. The roles of signal transducer and activator of transcription factor 3 in tumor angiogenesis. Oncotarget. 2017; 8:69139–61.
Article51. McGettrick AF, O’Neill LA. The role of HIF in immunity and inflammation. Cell Metab. 2020; 32:524–36.
Article52. Jun JC, Rathore A, Younas H, Gilkes D, Polotsky VY. Hypoxia-inducible factors and cancer. Curr Sleep Med Rep. 2017; 3:1–10.53. Dengler VL, Galbraith M, Espinosa JM. Transcriptional regulation by hypoxia inducible factors. Crit Rev Biochem Mol Biol. 2014; 49:1–15.
Article54. Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001; 292:468–72.
Article55. Harada H, Inoue M, Itasaka S, Hirota K, Morinibu A, Shinomiya K, et al. Cancer cells that survive radiation therapy acquire HIF-1 activity and translocate towards tumour blood vessels. Nat Commun. 2012; 3:783.
Article56. Holmquist-Mengelbier L, Fredlund E, Lofstedt T, Noguera R, Navarro S, Nilsson H, et al. Recruitment of HIF-1alpha and HIF-2alpha to common target genes is differentially regulated in neuroblastoma: HIF-2alpha promotes an aggressive phenotype. Cancer Cell. 2006; 10:413–23.57. Li S, Wen L, Hu X, Wei Q, Dong Z. HIF in nephrotoxicity during cisplatin chemotherapy: regulation, function and therapeutic potential. Cancers (Basel). 2021; 13:180.
Article58. Soni S, Padwad YS. HIF-1 in cancer therapy: two decade long story of a transcription factor. Acta Oncol. 2017; 56:503–15.59. Yan Y, He M, Zhao L, Wu H, Zhao Y, Han L, et al. A novel HIF-2alpha targeted inhibitor suppresses hypoxia-induced breast cancer stemness via SOD2-mtROS-PDI/GPR78-UPR(ER) axis. Cell Death Differ. 2022; 29:1769–89.
Article60. Albadari N, Deng S, Li W. The transcriptional factors HIF-1 and HIF-2 and their novel inhibitors in cancer therapy. Expert Opin Drug Discov. 2019; 14:667–82.61. Moreno Roig E, Groot AJ, Yaromina A, Hendrickx TC, Barbeau LM, Giuranno L, et al. HIF-1alpha and HIF-2alpha differently regulate the radiation sensitivity of NSCLC cells. Cells. 2019; 8:45.
Article62. Semenza GL. HIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations. J Clin Invest. 2013; 123:3664–71.
Article63. Nguyen TL, Duran RV. Prolyl hydroxylase domain enzymes and their role in cell signaling and cancer metabolism. Int J Biochem Cell Biol. 2016; 80:71–80.64. Sartori-Cintra AR, Mara CS, Argolo DL, Coimbra IB. Regulation of hypoxia-inducible factor-1alpha (HIF-1alpha) expression by interleukin-1beta (IL-1 beta), insulin-like growth factors I (IGF-I) and II (IGF-II) in human osteoarthritic chondrocytes. Clinics (Sao Paulo). 2012; 67:35–40.65. Suwa T, Kobayashi M, Nam JM, Harada H. Tumor microenvironment and radioresistance. Exp Mol Med. 2021; 53:1029–35.66. Obacz J, Pastorekova S, Vojtesek B, Hrstka R. Cross-talk between HIF and p53 as mediators of molecular responses to physiological and genotoxic stresses. Mol Cancer. 2013; 12:93.67. Kim W, Lee S, Seo D, Kim D, Kim K, Kim E, et al. Cellular stress responses in radiotherapy. Cells. 2019; 8:1105.
Article68. Zhu M, Yang M, Zhang J, Yin Y, Fan X, Zhang Y, et al. Immunogenic cell death induction by ionizing radiation. Front Immunol. 2021; 12:705361.69. Liu L, Ning X, Sun L, Shi Y, Han S, Guo C, et al. Involvement of MGr1-Ag/37LRP in the vincristine-induced HIF-1 expression in gastric cancer cells. Mol Cell Biochem. 2007; 303:151–60.70. Sia J, Szmyd R, Hau E, Gee HE. Molecular mechanisms of radiation-induced cancer cell death: a primer. Front Cell Dev Biol. 2020; 8:41.71. Spina CS, Drake CG. Mechanisms of immune modulation by radiation. Semin Radiat Oncol. 2021; 31:205–16.72. Zhai J, Gu X, Liu Y, Hu Y, Jiang Y, Zhang Z. Chemotherapeutic and targeted drugs-induced immunogenic cell death in cancer models and antitumor therapy: an update review. Front Pharmacol. 2023; 14:1152934.73. Bracci L, Schiavoni G, Sistigu A, Belardelli F. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ. 2014; 21:15–25.74. Gupta G, Borglum K, Chen H. Immunogenic cell death: a step ahead of autophagy in cancer therapy. J Cancer Immunol (Wilmington). 2021; 3:47–59.75. Tran CW, Gold MJ, Garcia-Batres C, Tai K, Elford AR, Himmel ME, et al. Hypoxia-inducible factor 1 alpha limits dendritic cell stimulation of CD8 T cell immunity. PLoS One. 2020; 15:e0244366.76. Chao MP, Jaiswal S, Weissman-Tsukamoto R, Alizadeh AA, Gentles AJ, Volkmer J, et al. Calreticulin is the dominant prophagocytic signal on multiple human cancers and is counterbalanced by CD47. Sci Transl Med. 2010; 2:63ra94.
Article77. Thepmalee C, Panya A, Junking M, Chieochansin T, Yenchitsomanus PT. Inhibition of IL-10 and TGF-beta receptors on dendritic cells enhances activation of effector T-cells to kill cholangiocarcinoma cells. Hum Vaccin Immunother. 2018; 14:1423–31.78. Lai JZ, Zhu YY, Ruan M, Chen L, Zhang QY. Local irradiation sensitized tumors to adoptive T cell therapy via enhancing the cross-priming, homing, and cytotoxicity of antigen-specific CD8 T cells. Front Immunol. 2019; 10:2857.79. Wen Q, Han T, Wang Z, Jiang S. Role and mechanism of programmed death-ligand 1 in hypoxia-induced liver cancer immune escape. Oncol Lett. 2020; 19:2595–601.80. Sethumadhavan S, Silva M, Philbrook P, Nguyen T, Hatfield SM, Ohta A, et al. Hypoxia and hypoxia-inducible factor (HIF) downregulate antigen-presenting MHC class I molecules limiting tumor cell recognition by T cells. PLoS One. 2017; 12:e0187314.81. Baginska J, Viry E, Paggetti J, Medves S, Berchem G, Moussay E, et al. The critical role of the tumor microenvironment in shaping natural killer cell-mediated anti-tumor immunity. Front Immunol. 2013; 4:490.82. Fung AS, Lee C, Yu M, Tannock IF. The effect of chemotherapeutic agents on tumor vasculature in subcutaneous and orthotopic human tumor xenografts. BMC Cancer. 2015; 15:112.83. Lin N, Simon MC. Hypoxia-inducible factors: key regulators of myeloid cells during inflammation. J Clin Invest. 2016; 126:3661–71.84. Westendorf AM, Skibbe K, Adamczyk A, Buer J, Geffers R, Hansen W, et al. Hypoxia enhances immunosuppression by inhibiting CD4+ effector T cell function and promoting Treg activity. Cell Physiol Biochem. 2017; 41:1271–84.85. Bowser JL, Lee JW, Yuan X, Eltzschig HK. The hypoxia-adenosine link during inflammation. J Appl Physiol (1985). 2017; 123:1303–20.86. Devi VJ, Radhika A, Biju PG. Adenosine receptor activation promotes macrophage class switching from LPS-induced acute inflammatory M1 to anti-inflammatory M2 phenotype. Immunobiology. 2023; 228:152362.87. Zhang H, Lu H, Xiang L, Bullen JW, Zhang C, Samanta D, et al. HIF-1 regulates CD47 expression in breast cancer cells to promote evasion of phagocytosis and maintenance of cancer stem cells. Proc Natl Acad Sci U S A. 2015; 112:E6215–23.88. Samanta D, Park Y, Ni X, Li H, Zahnow CA, Gabrielson E, et al. Chemotherapy induces enrichment of CD47(+)/CD73(+)/PDL1(+) immune evasive triple-negative breast cancer cells. Proc Natl Acad Sci U S A. 2018; 115:E1239–48.89. Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, et al. PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med. 2014; 211:781–90.90. Ruf M, Moch H, Schraml P. PD-L1 expression is regulated by hypoxia inducible factor in clear cell renal cell carcinoma. Int J Cancer. 2016; 139:396–403.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hypoxia-Inducible Factor-1 Alpha Stabilization in Human Macrophages during Leishmania major Infection Is Impaired by Parasite Virulence
- ACY-241, a histone deacetylase 6 inhibitor, suppresses the epithelial–mesenchymal transition in lung cancer cells by downregulating hypoxia-inducible factor-1 alpha
- Is Biochemical Follow Up Possible in Peripheral Arterial Disease Treatment: Hypoxia Inducible Factor-1 Alpha?
- Myocardin Reverses Hypoxia-Inducible Factor-1α Mediated Phenotypic Modulation of Corpus Cavernosum Smooth Muscle Cells in Hypoxia Induced by Cobalt Chloride
- Anti-Tumor Effect of IDF-11774, an Inhibitor of Hypoxia-Inducible Factor-1, on Melanoma