Cancer Res Treat.
2021 Oct;53(4):1166-1173. 10.4143/crt.2021.091.
Use of Gemcitabine plus Carboplatin is Associated with Poor Outcomes in Urothelial Carcinoma Patients with Chronic Kidney Disease Stage 4-5
- Affiliations
-
- 1Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 2Division of Hematology-Oncology, Department of Internal Medicine, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan, Korea
- 3Division of Medical Oncology, Department of Internal Medicine, Gachon University Gil Medical Center, Incheon, Korea
- KMID: 2521591
- DOI: http://doi.org/10.4143/crt.2021.091
Abstract
- Purpose
This study aimed to investigate the clinical outcomes with gemcitabine-carboplatin (GCb), the standard treatment for patients with advanced urothelial carcinoma (UC) who are ineligible for cisplatin-based regimens, in advanced UC patients with a glomerular filtration rate (GFR) < 30 mL/min.
Materials and Methods
A retrospective cohort study involving GCb-treated advanced UC patients with GFR < 60 mL/min (n=89) was performed. Clinical outcomes were compared between subgroups with GFR < 30 mL/min and GFR ≥ 30 mL/min but < 60 mL/min.
Results
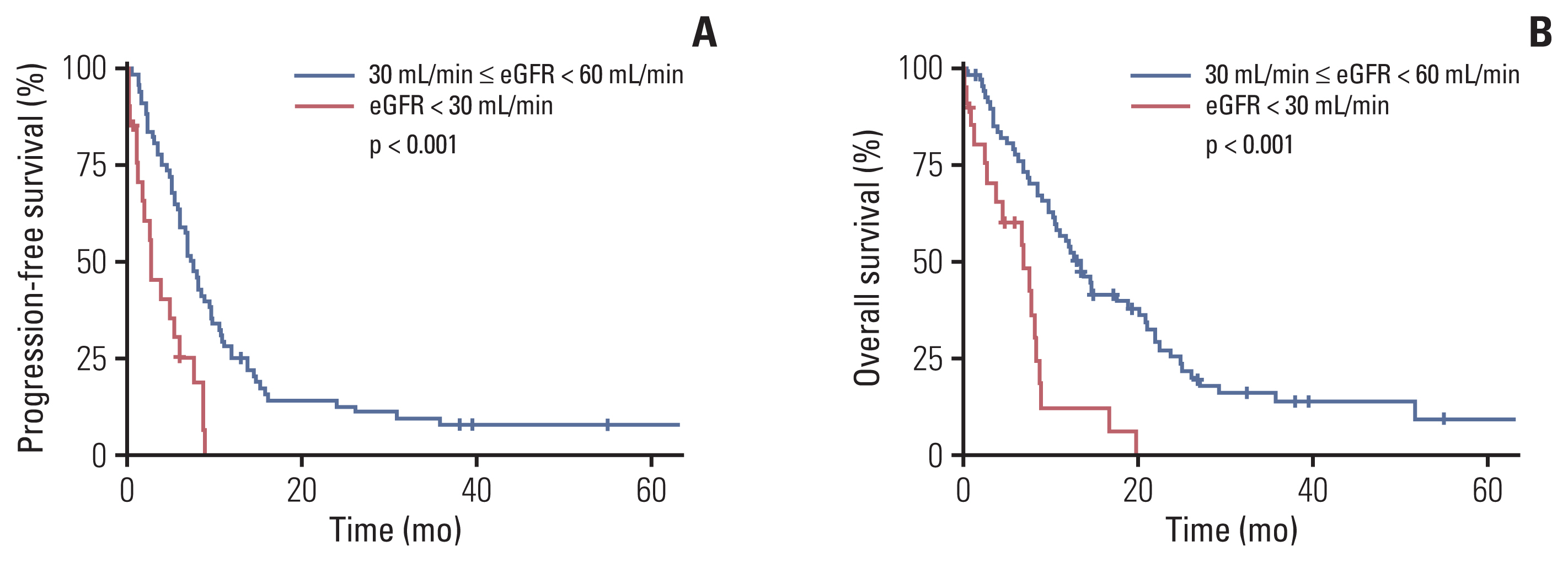
Most baseline characteristics were comparable between the two subgroups. Patients with GFR < 30 mL/min had a significantly lower objective response rate (12.5%) compared to those with higher GFR levels (56.7%) (p=0.004). The number of GCb cycles was significantly lower in patients with GFR < 30 mL/min (median 2 cycles) than in those with higher GFR levels (median 6 cycles) (p=0.002). Compared to those with GFR ≥ 30 mL/min but < 60 mL/min, patients with GFR < 30 mL/min showed significantly worse progression-free survival (PFS) and overall survival (OS) (p < 0.001 for both). Further stratification of patient subgroups according to their GFR (i.e., GFR ≥ 45 mL/min but < 60 mL/min vs. GFR ≥ 30 mL/min but < 45 mL/min vs. GFR < 30 mL/min) revealed significantly different PFS and OS (p < 0.001 for both).
Conclusion
The use of GCb is discouraged in advanced UC patients with GFR < 30 mL/min. Alternative therapeutic approaches with better efficacy are warranted for these patients.
Keyword
Figure
Reference
-
References
1. Koufopoulou M, Miranda PA, Kazmierska P, Deshpande S, Gaitonde P. Clinical evidence for the first-line treatment of advanced urothelial carcinoma: current paradigms and emerging treatment options. Cancer Treat Rev. 2020; 89:102072.
Article2. von der Maase H, Hansen SW, Roberts JT, Dogliotti L, Oliver T, Moore MJ, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000; 18:3068–77.
Article3. von der Maase H, Sengelov L, Roberts JT, Ricci S, Dogliotti L, Oliver T, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005; 23:4602–8.
Article4. Bellmunt J, Eigl BJ, Senkus E, Loriot Y, Twardowski P, Castellano D, et al. Borealis-1: a randomized, first-line, placebo-controlled, phase II study evaluating apatorsen and chemotherapy for patients with advanced urothelial cancer. Ann Oncol. 2017; 28:2481–8.
Article5. Galsky MD, Hahn NM, Rosenberg J, Sonpavde G, Hutson T, Oh WK, et al. Treatment of patients with metastatic urothelial cancer “unfit” for cisplatin-based chemotherapy. J Clin Oncol. 2011; 29:2432–8.
Article6. Bukhari N, Al-Shamsi HO, Azam F. Update on the treatment of metastatic urothelial carcinoma. ScientificWorldJournal. 2018; 2018:5682078.
Article7. De Santis M, Bellmunt J, Mead G, Kerst JM, Leahy M, Maroto P, et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J Clin Oncol. 2012; 30:191–9.
Article8. Na SY, Sung JY, Chang JH, Kim S, Lee HH, Park YH, et al. Chronic kidney disease in cancer patients: an independent predictor of cancer-specific mortality. Am J Nephrol. 2011; 33:121–30.
Article9. Park I, Kim BS, Lim HY, Kim HJ, Lee HJ, Choi YJ, et al. Gemcitabine plus carboplatin versus gemcitabine plus oxaliplatin in cisplatin-unfit patients with advanced urothelial carcinoma: a randomised phase II study (COACH, KCSG GU10-16). Eur J Cancer. 2020; 127:183–90.
Article10. Havrilesky LJ, Reiner M, Morrow PK, Watson H, Crawford J. A review of relative dose intensity and survival in patients with metastatic solid tumors. Crit Rev Oncol Hematol. 2015; 93:203–10.
Article11. Beumer JH, Inker LA, Levey AS. Improving carboplatin dosing based on estimated GFR. Am J Kidney Dis. 2018; 71:163–5.12. Merchan JR, Jhaveri KD. Chemotherapy nephrotoxicity and dose modification in patients with kidney impairment: conventional cytotoxic agents [Internet]. Waltham, MA: UpToDate;2020. [cited 2020 Dec 20]. Available from: https://www.uptodate.com/contents/chemotherapy-nephrotoxicity-and-dose-modification-in-patients-with-kidney-impairment-conventional-cytotoxic-agents .13. Launay-Vacher V, Janus N, Deray G. Renal insufficiency and cancer treatments. ESMO Open. 2016; 1:e000091.
Article14. Iff S, Craig JC, Turner R, Chapman JR, Wang JJ, Mitchell P, et al. Reduced estimated GFR and cancer mortality. Am J Kidney Dis. 2014; 63:23–30.
Article15. Perazella MA, Shirali AC. Immune checkpoint inhibitor nephrotoxicity: what do we know and what should we do? Kidney Int. 2020; 97:62–74.
Article16. Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017; 376:1015–26.
Article17. Fradet Y, Bellmunt J, Vaughn DJ, Lee JL, Fong L, Vogelzang NJ, et al. Randomized phase III KEYNOTE-045 trial of pembrolizumab versus paclitaxel, docetaxel, or vinflunine in recurrent advanced urothelial cancer: results of >2 years of follow-up. Ann Oncol. 2019; 30:970–6.18. Powles T, van der Heijden MS, Castellano D, Galsky MD, Loriot Y, Petrylak DP, et al. Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2020; 21:1574–88.19. Galsky MD, Arija JA, Bamias A, Davis ID, De Santis M, Kikuchi E, et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2020; 395:1547–57.
Article20. Alva A, Csoszi T, Ozguroglu M, Matsubara N, Geczi L, Cheng SY, et al. LBA23 Pembrolizumab (P) combined with chemotherapy (C) vs C alone as first-line (1L) therapy for advanced urothelial carcinoma (UC): KEYNOTE-361. Ann Oncol. 2020; 31(Suppl 4):S1155.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Chemotherapy in Advanced Urothelial Carcinoma
- A case of gemcitabine-induced thrombotic microangiopathy in a urothelial tumor patient with a single kidney
- A Phase II Study with Gemcitabine and Carboplatin in Patients with Advanced Non-small Cell Lung Cancer
- Gemcitabine and Carboplatin Combination Chemotherapy for Elderly Patients with Advanced Non-small Cell Lung Cancer: A Feasibility Study
- Sarcomatoid Urothelial Carcinoma of the Renal Pelvis with Extremely Aggressive Clinical Behavior