Korean J Transplant.
2021 Sep;35(3):149-160. 10.4285/kjt.21.0014.
Impact of delayed graft function on clinical outcomes in highly sensitized patients after deceased-donor kidney transplantation
- Affiliations
-
- 1Division of Nephrology, Department of Internal Medicine, Daegu Catholic University School of Medicine, Daegu, Korea
- 2Transplantation Research Center, Division of Nephrology, Department of Internal Medicine, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 3Division of Nephrology, Department of Internal Medicine, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 4Division of Nephrology, Department of Internal Medicine, Uijeongbu St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Uijeongbu, Korea
- 5Division of Nephrology, Department of Internal Medicine, Keimyung University School of Medicine, Daegu, Korea
- KMID: 2520676
- DOI: http://doi.org/10.4285/kjt.21.0014
Abstract
- Background
We investigated whether the development of delayed graft function (DGF) in pre-sensitized patients affects the clinical outcomes after deceased-donor kidney transplantation (DDKT).
Methods
The study included 709 kidney transplant recipients (KTRs) from three transplant centers. We divided KTRs into four subgroups (highly sensitized DGF, highly sensitized non-DGF, low-sensitized DGF, and low-sensitized non-DGF) according to panel reactive antibody level of 50%, or DGF development. We compared post-transplant clinical outcomes among the four subgroups.
Results
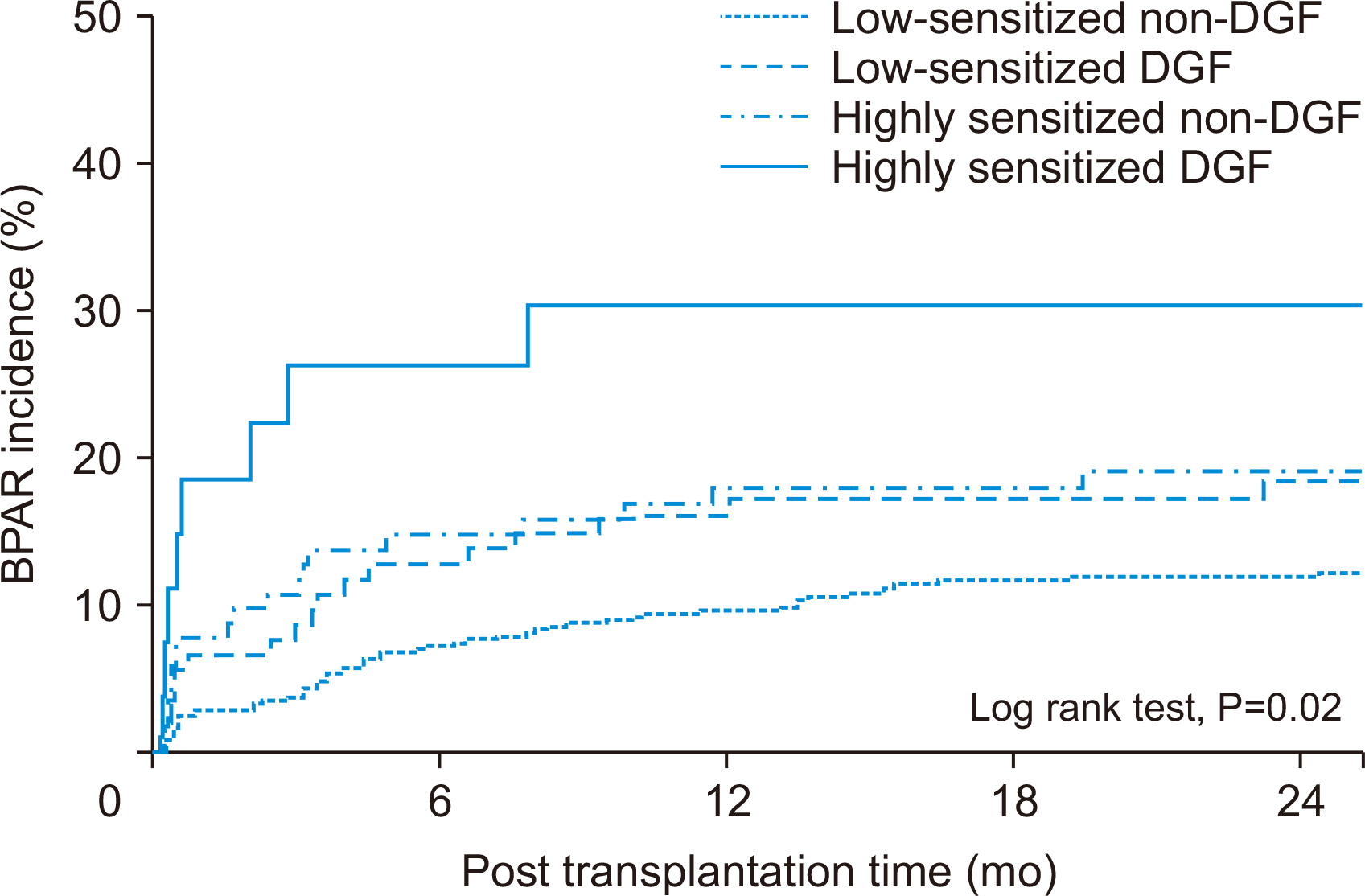
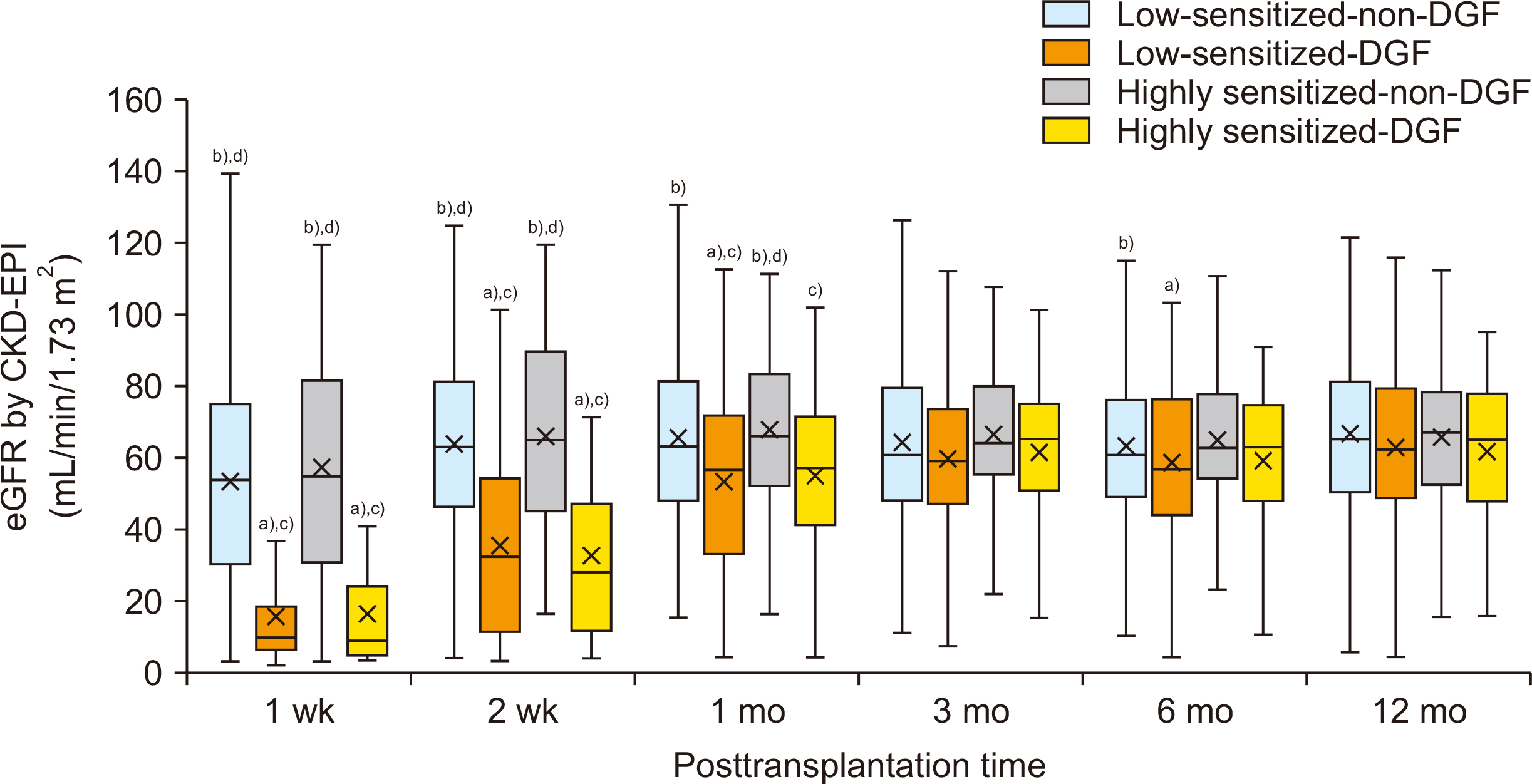
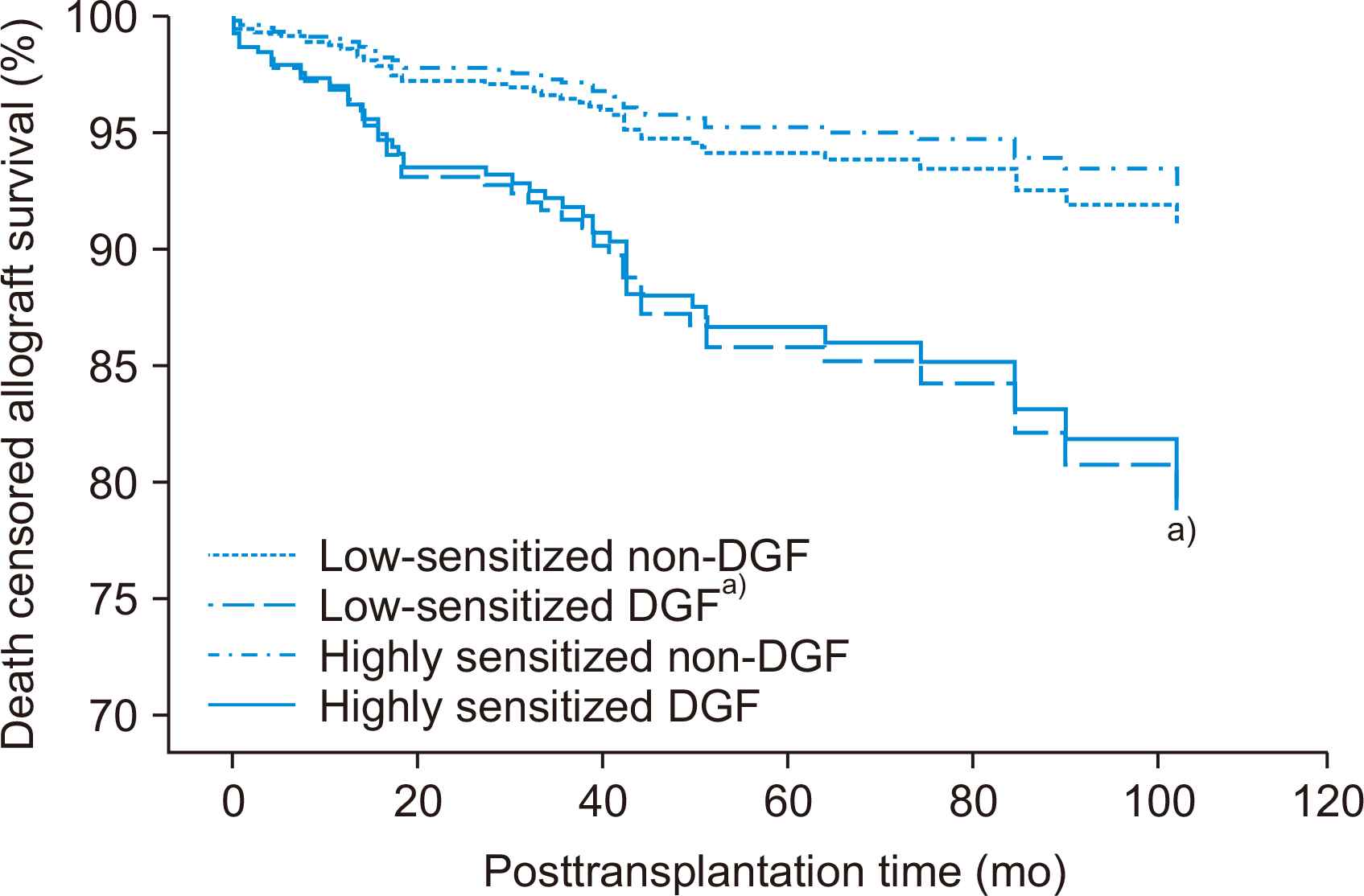
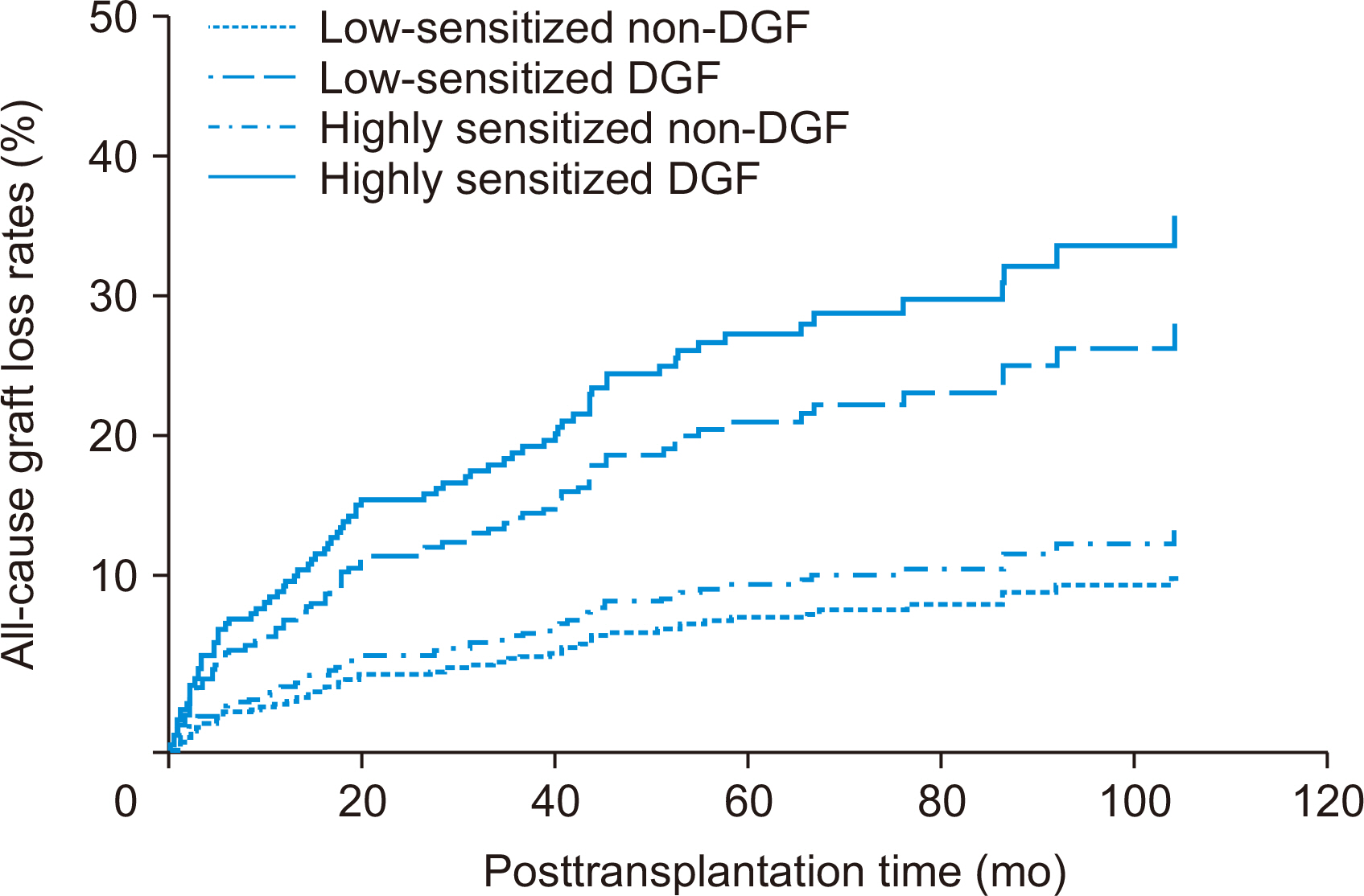
Incidence of biopsy-proven acute rejection (BPAR) was higher in two highly sensitized subgroups than in low-sensitized subgroups. It tended to be higher in highly sensitized DGF subgroups than in the highly sensitized non-DGF subgroups. In addition, the highly sensitized DGF subgroup showed the highest risk for BPAR (hazard ratio, 3.051;P=0.005) and independently predicted BPAR. Allograft function was lower in the two DGF subgroups than in the non-DGF subgroup until one month after transplantation, but thereafter it was similar. Death-censored graft loss rates and patient mortality tended to be low when DGF developed, but it did not reach statistical significance.
Conclusions
DGF development in highly sensitized patients increases the risk for BPAR in DDKT compared with patients without DGF, suggesting the need for strict monitoring and management of such cases.
Figure
Reference
-
1. Yarlagadda SG, Coca SG, Garg AX, Doshi M, Poggio E, Marcus RJ, et al. 2008; Marked variation in the definition and diagnosis of delayed graft function: a systematic review. Nephrol Dial Transplant. 23:2995–3003. DOI: 10.1093/ndt/gfn158. PMID: 18408075. PMCID: PMC2727302.
Article2. Irish WD, Ilsley JN, Schnitzler MA, Feng S, Brennan DC. 2010; A risk prediction model for delayed graft function in the current era of deceased donor renal transplantation. Am J Transplant. 10:2279–86. DOI: 10.1111/j.1600-6143.2010.03179.x. PMID: 20883559.
Article3. Siedlecki A, Irish W, Brennan DC. 2011; Delayed graft function in the kidney transplant. Am J Transplant. 11:2279–96. DOI: 10.1111/j.1600-6143.2011.03754.x. PMID: 21929642. PMCID: PMC3280444.
Article4. Ponticelli C. 2014; Ischaemia-reperfusion injury: a major protagonist in kidney transplantation. Nephrol Dial Transplant. 29:1134–40. DOI: 10.1093/ndt/gft488. PMID: 24335382.
Article5. Yarlagadda SG, Coca SG, Formica RN Jr, Poggio ED, Parikh CR. 2009; Association between delayed graft function and allograft and patient survival: a systematic review and meta-analysis. Nephrol Dial Transplant. 24:1039–47. DOI: 10.1093/ndt/gfn667. PMID: 19103734.
Article6. Wu WK, Famure O, Li Y, Kim SJ. 2015; Delayed graft function and the risk of acute rejection in the modern era of kidney transplantation. Kidney Int. 88:851–8. DOI: 10.1038/ki.2015.190. PMID: 26108067.
Article7. Dikow R, Becker LE, Schaier M, Waldherr R, Gross ML, Zeier M. 2010; In renal transplants with delayed graft function chemokines and chemokine receptor expression predict long-term allograft function. Transplantation. 90:771–6. DOI: 10.1097/TP.0b013e3181f009ef. PMID: 20697328.
Article8. Viglietti D, Abboud I, Hill G, Vernerey D, Nochy D, Antoine C, et al. 2015; Kidney allograft fibrosis after transplantation from uncontrolled circulatory death donors. Transplantation. 99:409–15. DOI: 10.1097/TP.0000000000000228. PMID: 25222117.
Article9. Jordan SC, Pescovitz MD. 2006; Presensitization: the problem and its management. Clin J Am Soc Nephrol. 1:421–32. DOI: 10.2215/CJN.01651105. PMID: 17699241.
Article10. Filippone EJ, Farber JL. 2015; Humoral immune response and allograft function in kidney transplantation. Am J Kidney Dis. 66:337–47. DOI: 10.1053/j.ajkd.2015.03.033. PMID: 25987262.
Article11. Loupy A, Hill GS, Jordan SC. 2012; The impact of donor-specific anti-HLA antibodies on late kidney allograft failure. Nat Rev Nephrol. 8:348–57. DOI: 10.1038/nrneph.2012.81. PMID: 22508180.
Article12. Sethi S, Najjar R, Peng A, Mirocha J, Vo A, Bunnapradist S, et al. 2019; Allocation of the highest quality kidneys and transplant outcomes under the new kidney allocation system. Am J Kidney Dis. 73:605–14. DOI: 10.1053/j.ajkd.2018.12.036. PMID: 30929853.
Article13. Montgomery RA, Lonze BE, King KE, Kraus ES, Kucirka LM, Locke JE, et al. 2011; Desensitization in HLA-incompatible kidney recipients and survival. N Engl J Med. 365:318–26. DOI: 10.1056/NEJMoa1012376. PMID: 21793744.
Article14. Marfo K, Lu A, Ling M, Akalin E. 2011; Desensitization protocols and their outcome. Clin J Am Soc Nephrol. 6:922–36. DOI: 10.2215/CJN.08140910. PMID: 21441131.
Article15. Hwang SD, Chung BH, Oh EJ, Choi BS, Park CW, Kim YS, et al. 2015; Effect of pretransplant rituximab use on posttransplant clinical outcomes in patients with high panel reactive antibody scores. Nephron. 130:239–44. DOI: 10.1159/000435924. PMID: 26182858.
Article16. Vo AA, Petrozzino J, Yeung K, Sinha A, Kahwaji J, Peng A, et al. 2013; Efficacy, outcomes, and cost-effectiveness of desensitization using IVIG and rituximab. Transplantation. 95:852–8. DOI: 10.1097/TP.0b013e3182802f88. PMID: 23511212.
Article17. Lieber SR, Perez FV, Tabossi MR, Persoli LB, Marques SB, Mazzali M, et al. 2007; Effect of panel-reactive antibody in predicting crossmatch selection of cadaveric kidney recipients. Transplant Proc. 39:429–31. DOI: 10.1016/j.transproceed.2007.01.045. PMID: 17362748.
Article18. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. 2009; A new equation to estimate glomerular filtration rate. Ann Intern Med. 150:604–12. DOI: 10.7326/0003-4819-150-9-200905050-00006. PMID: 19414839. PMCID: PMC2763564.
Article19. Sis B, Mengel M, Haas M, Colvin RB, Halloran PF, Racusen LC, et al. 2010; Banff '09 meeting report: antibody mediated graft deterioration and implementation of Banff working groups. Am J Transplant. 10:464–71. DOI: 10.1111/j.1600-6143.2009.02987.x. PMID: 20121738.
Article20. Metzger RA, Delmonico FL, Feng S, Port FK, Wynn JJ, Merion RM. 2003; Expanded criteria donors for kidney transplantation. Am J Transplant. 3 Suppl 4:114–25. DOI: 10.1034/j.1600-6143.3.s4.11.x. PMID: 12694055.
Article21. Noël C, Abramowicz D, Durand D, Mourad G, Lang P, Kessler M, et al. 2009; Daclizumab versus antithymocyte globulin in high-immunological-risk renal transplant recipients. J Am Soc Nephrol. 20:1385–92. DOI: 10.1681/ASN.2008101037. PMID: 19470677. PMCID: PMC2689909.
Article22. van den Hoogen MW, Kamburova EG, Baas MC, Steenbergen EJ, Florquin S, M Koenen HJ, et al. 2015; Rituximab as induction therapy after renal transplantation: a randomized, double-blind, placebo-controlled study of efficacy and safety. Am J Transplant. 15:407–16. DOI: 10.1111/ajt.13052. PMID: 25612493.
Article23. Shoskes DA, Parfrey NA, Halloran PF. 1990; Increased major histocompatibility complex antigen expression in unilateral ischemic acute tubular necrosis in the mouse. Transplantation. 49:201–7. DOI: 10.1097/00007890-199001000-00045. PMID: 2105546.
Article24. Li K, Fazekasova H, Wang N, Sagoo P, Peng Q, Khamri W, et al. 2011; Expression of complement components, receptors and regulators by human dendritic cells. Mol Immunol. 48:1121–7. DOI: 10.1016/j.molimm.2011.02.003. PMID: 21397947. PMCID: PMC3084445.
Article25. van Werkhoven MB, Damman J, van Dijk MC, Daha MR, de Jong IJ, Leliveld A, et al. 2013; Complement mediated renal inflammation induced by donor brain death: role of renal C5a-C5aR interaction. Am J Transplant. 13:875–82. DOI: 10.1111/ajt.12130. PMID: 23398742.
Article26. Crabtree GR. 1989; Contingent genetic regulatory events in T lymphocyte activation. Science. 243:355–61. DOI: 10.1126/science.2783497. PMID: 2783497.
Article27. Meier-Kriesche HU, Ojo A, Hanson J, Cibrik D, Lake K, Agodoa LY, et al. 2000; Increased immunosuppressive vulnerability in elderly renal transplant recipients. Transplantation. 69:885–9. DOI: 10.1097/00007890-200003150-00037. PMID: 10755545.28. Tullius SG, Milford E. 2011; Kidney allocation and the aging immune response. N Engl J Med. 364:1369–70. DOI: 10.1056/NEJMc1103007. PMID: 21410395.
Article29. Martins PN, Pratschke J, Pascher A, Fritsche L, Frei U, Neuhaus P, et al. 2005; Age and immune response in organ transplantation. Transplantation. 79:127–32. DOI: 10.1097/01.TP.0000146258.79425.04. PMID: 15665758.
Article30. Weiskopf D, Weinberger B, Grubeck-Loebenstein B. 2009; The aging of the immune system. Transpl Int. 22:1041–50. DOI: 10.1111/j.1432-2277.2009.00927.x. PMID: 19624493.
Article31. Wissing KM, Fomegné G, Broeders N, Ghisdal L, Hoang AD, Mikhalski D, et al. 2008; HLA mismatches remain risk factors for acute kidney allograft rejection in patients receiving quadruple immunosuppression with anti-interleukin-2 receptor antibodies. Transplantation. 85:411–6. DOI: 10.1097/TP.0b013e31816349b5. PMID: 18322434.
Article32. Foster BJ, Dahhou M, Zhang X, Platt RW, Smith JM, Hanley JA. 2014; Impact of HLA mismatch at first kidney transplant on lifetime with graft function in young recipients. Am J Transplant. 14:876–85. DOI: 10.1111/ajt.12643. PMID: 24612783.
Article33. Kosmoliaptsis V, Mallon DH, Chen Y, Bolton EM, Bradley JA, Taylor CJ. 2016; Alloantibody responses after renal transplant failure can be better predicted by donor-recipient HLA amino acid sequence and physicochemical disparities than conventional HLA matching. Am J Transplant. 16:2139–47. DOI: 10.1111/ajt.13707. PMID: 26755448. PMCID: PMC5021128.
Article34. Vlad G, Ho EK, Vasilescu ER, Colovai AI, Stokes MB, Markowitz GS, et al. 2009; Relevance of different antibody detection methods for the prediction of antibody-mediated rejection and deceased-donor kidney allograft survival. Hum Immunol. 70:589–94. DOI: 10.1016/j.humimm.2009.04.018. PMID: 19375470.
Article35. Wehmeier C, Hönger G, Cun H, Amico P, Hirt-Minkowski P, Georgalis A, et al. 2017; Donor specificity but not broadness of sensitization is associated with antibody-mediated rejection and graft loss in renal allograft recipients. Am J Transplant. 17:2092–102. DOI: 10.1111/ajt.14247. PMID: 28245084.
Article36. Tambur AR, Herrera ND, Haarberg KM, Cusick MF, Gordon RA, Leventhal JR, et al. 2015; Assessing antibody strength: comparison of MFI, C1q, and titer information. Am J Transplant. 15:2421–30. DOI: 10.1111/ajt.13295. PMID: 25930984.
Article37. Shamali A, Kassimatis T, Phillips BL, Burton H, Kessaris N, Callaghan C. 2019; Duration of delayed graft function and outcomes after kidney transplantation from controlled donation after circulatory death donors: a retrospective study. Transpl Int. 32:635–45. DOI: 10.1111/tri.13403. PMID: 30685880.38. Brennan DC, Daller JA, Lake KD, Cibrik D, Del Castillo D. Thymoglobulin Induction Study Group. 2006; Rabbit antithymocyte globulin versus basiliximab in renal transplantation. N Engl J Med. 355:1967–77. DOI: 10.1056/NEJMoa060068. PMID: 17093248.
Article39. Jeong ES, Lee KW, Kim SJ, Yoo HJ, Kim KA, Park JB. 2019; Comparison of clinical outcomes of deceased donor kidney transplantations, with a focus on three induction therapies. Korean J Transplant. 33:118–27. DOI: 10.4285/jkstn.2019.33.4.118.
Article40. Tapiawala SN, Tinckam KJ, Cardella CJ, Schiff J, Cattran DC, Cole EH, et al. 2010; Delayed graft function and the risk for death with a functioning graft. J Am Soc Nephrol. 21:153–61. DOI: 10.1681/ASN.2009040412. PMID: 19875806. PMCID: PMC2799285.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Preliminary Study to Revise the Marginal Donor Criteria of KONOS in Deceased Donor Kidney Transplantation
- Thrombotic microangiopathy, rare cause of deceased donor acute kidney injury: is a donor biopsy necessary before donation?
- Evaluation and Utilization of Expanded Criteria Dornor
- The Use of Non-Heart Beating Donors to Expand the Donor Pool
- Back on track: outcomes of deceased donor kidney recipients at national kidney and transplant institute during the COVID-19 pandemic