Blood Res.
2021 Sep;56(3):184-196. 10.5045/br.2021.2021107.
Development and validation of a comorbidity index for predicting survival outcomes after allogeneic stem cell transplantation in adult patients with acute leukemia: a Korean nationwide cohort study
- Affiliations
-
- 1Department of Hematology, Catholic Hematology Hospital, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Kore
- 2Leukemia Research Institute, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2520612
- DOI: http://doi.org/10.5045/br.2021.2021107
Abstract
- Background
Allogeneic hematopoietic stem cell transplantation (alloSCT) is a potentially curative treatment option for acute leukemia. We aimed to identify the comorbidity factors affecting survival outcomes after alloSCT and develop a new comorbidity index tool for predicting overall survival (OS).
Methods
A Korean nationwide cohort of 3,809 adults with acute leukemia treated with alloSCT between January 2002 and December 2018 was analyzed as the development cohort. A retrospective cohort comprising 313 consecutive adults with acute leukemia who underwent alloSCT between January 2019 and April 2020 was analyzed as the validation cohort.
Results
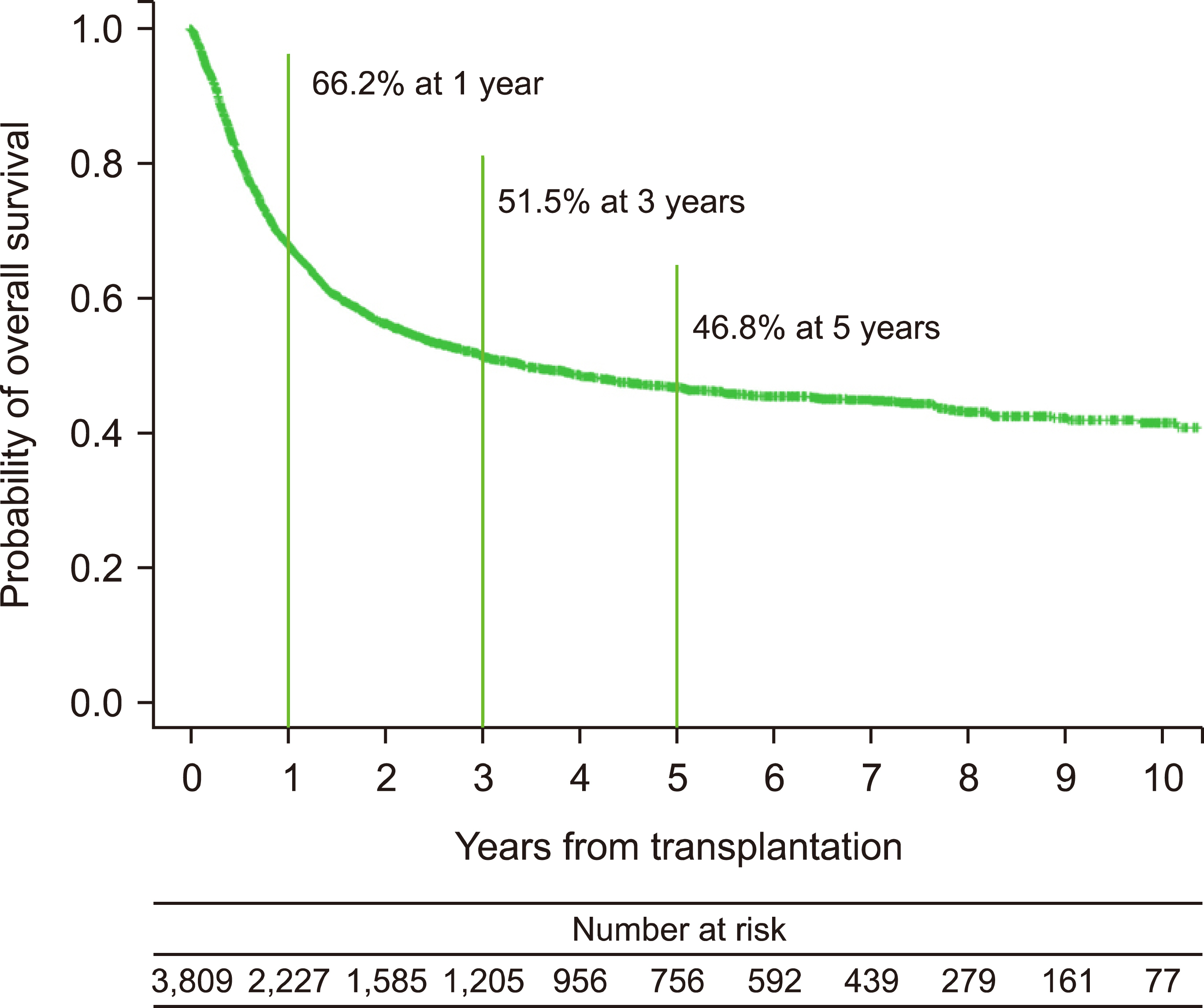
In the development cohort, advanced age, male sex, and comorbidities such as previous non-hematologic malignancy, hypertension, and coronary or cerebral vascular disease were significantly related to poor OS. Subsequently, a new comorbidity scoring system was developed, and risk groups were created, which included the low-risk (score ≤0.17), intermediate-risk (0.17< score ≤0.4), high-risk (0.4< score ≤0.55), and very high-risk (score >0.55) groups. The 1-year OS rates were discriminatively estimated at 73.5%, 66.2%, 61.9%, and 50.9% in the low-risk, intermediate-risk, high-risk, and very high-risk groups in the development cohort, respectively (P <0.001). The developed scoring system yielded discriminatively different 1-year OS rates and 1-year incidence of non-relapse mortality according to the risk group (P =0.085 and P =0.018, respectively). Furthermore, the developed model showed an acceptable performance for predicting 1-year non-relapse mortality with an area under the curve of 0.715.
Conclusion
The newly developed predictive scoring system could be a simple and reliable tool helping clinicians to assess risk of alloSCT in adults with acute leukemia.
Keyword
Figure
Reference
-
1. Mukherjee S, Sekeres MA. 2019; Novel therapies in acute myeloid leukemia. Semin Oncol Nurs. 35:150955. DOI: 10.1016/j.soncn.2019.150955. PMID: 31759818.
Article2. Samra B, Jabbour E, Ravandi F, Kantarjian H, Short NJ. 2020; Evolving therapy of adult acute lymphoblastic leukemia: state-of-the-art treatment and future directions. J Hematol Oncol. 13:70. DOI: 10.1186/s13045-020-00905-2. PMID: 32503572. PMCID: PMC7275444.
Article3. Tallman MS, Wang ES, Altman JK, et al. 2019; Acute myeloid leukemia, version 3.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 17:721–49. DOI: 10.6004/jnccn.2019.0028. PMID: 31200351.4. Yoon JH, Kim HJ, Park SS, et al. 2017; Long-term clinical outcomes of hematopoietic cell transplantation for intermediate-to-poor-risk acute myeloid leukemia during first remission according to available donor types. Oncotarget. 8:41590–604. DOI: 10.18632/oncotarget.15295. PMID: 28206975. PMCID: PMC5522252.
Article5. Yoon JH, Yhim HY, Kwak JY, et al. 2016; Minimal residual disease-based effect and long-term outcome of first-line dasatinib combined with chemotherapy for adult Philadelphia chromosome-positive acute lymphoblastic leukemia. Ann Oncol. 27:1081–8. DOI: 10.1093/annonc/mdw123. PMID: 26951627.
Article6. Brown PA, Wieduwilt M, Logan A, et al. 2019; Guidelines insights: acute lymphoblastic leukemia, version 1.2019. J Natl Compr Canc Netw. 17:414–23. DOI: 10.6004/jnccn.2019.0024. PMID: 31085755.7. Bacigalupo A, Sormani MP, Lamparelli T, et al. 2004; Reducing transplant-related mortality after allogeneic hematopoietic stem cell transplantation. Haematologica. 89:1238–47. PMID: 15477210.8. Sorror ML, Maris MB, Storb R, et al. 2005; Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 106:2912–9. DOI: 10.1182/blood-2005-05-2004. PMID: 15994282. PMCID: PMC1895304.
Article9. Sorror ML, Storb RF, Sandmaier BM, et al. 2014; Comorbidity-age index: a clinical measure of biologic age before allogeneic hematopoietic cell transplantation. J Clin Oncol. 32:3249–56. DOI: 10.1200/JCO.2013.53.8157. PMID: 25154831. PMCID: PMC4178523.
Article10. Charlson M, Szatrowski TP, Peterson J, Gold J. 1994; Validation of a combined comorbidity index. J Clin Epidemiol. 47:1245–51. DOI: 10.1016/0895-4356(94)90129-5. PMID: 7722560.
Article11. Kim DS. 2010; Introduction: health of the health care system in Korea. Soc Work Public Health. 25:127–41. DOI: 10.1080/19371910903070333. PMID: 20391257.
Article12. Wang SM, Park SS, Park SH, et al. 2020; Pre-transplant depression decreased overall survival of patients receiving allogeneic hematopoietic stem cell transplantation: a nationwide cohort study. Sci Rep. 10:15265. DOI: 10.1038/s41598-020-71208-2. PMID: 32943660. PMCID: PMC7499172.
Article13. Byun JM, Lee J, Shin SJ, Kang M, Yoon SS, Koh Y. 2018; Busulfan plus melphalan versus high-dose melphalan as conditioning regimens in autologous stem cell transplantation for newly diagnosed multiple myeloma. Blood Res. 53:105–9. DOI: 10.5045/br.2018.53.2.105. PMID: 29963515. PMCID: PMC6021568.
Article14. Kong SG, Jeong S, Lee S, Jeong JY, Kim DJ, Lee HS. 2021; Early transplantation-related mortality after allogeneic hematopoietic cell transplantation in patients with acute leukemia. BMC Cancer. 21:177. DOI: 10.1186/s12885-021-07897-3. PMID: 33602150. PMCID: PMC7891151.
Article15. Wang SM, Park SS, Park SH, et al. 2021; Pre-transplant dementia is associated with poor survival after hematopoietic stem cell transplantation: a nationwide cohort study with propensity score matched control. Clin Psychopharmacol Neurosci. 19:294–302. DOI: 10.9758/cpn.2021.19.2.294. PMID: 33888658. PMCID: PMC8077055.
Article16. Mandrekar JN. 2010; Receiver operating characteristic curve in diagnostic test assessment. J Thorac Oncol. 5:1315–6. DOI: 10.1097/JTO.0b013e3181ec173d. PMID: 20736804.
Article17. Sorror ML, Giralt S, Sandmaier BM, et al. 2007; Hematopoietic cell transplantation specific comorbidity index as an outcome predictor for patients with acute myeloid leukemia in first remission: combined FHCRC and MDACC experiences. Blood. 110:4606–13. DOI: 10.1182/blood-2007-06-096966. PMID: 17873123. PMCID: PMC2234788.18. Maruyama D, Fukuda T, Kato R, et al. 2007; Comparable antileukemia/lymphoma effects in nonremission patients undergoing allogeneic hematopoietic cell transplantation with a conventional cytoreductive or reduced-intensity regimen. Biol Blood Marrow Transplant. 13:932–41. DOI: 10.1016/j.bbmt.2007.04.004. PMID: 17640597.
Article19. Park SS, Jeon YW, Min GJ, et al. 2019; Graft-versus-host disease-free, relapse-free survival after allogeneic stem cell transplantation for myelodysplastic syndrome. Biol Blood Marrow Transplant. 25:63–72. DOI: 10.1016/j.bbmt.2018.08.004. PMID: 30103018.
Article20. Nakaya A, Mori T, Tanaka M, et al. 2014; Does the hematopoietic cell transplantation specific comorbidity index (HCT-CI) predict transplantation outcomes? A prospective multicenter validation study of the Kanto Study Group for Cell Therapy. Biol Blood Marrow Transplant. 20:1553–9. DOI: 10.1016/j.bbmt.2014.06.005. PMID: 25034961.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Ovarian-Relapse Sparing of the Marrow in a Patient with Acute T Cell Lymphoblastic Leukemia after Allogeneic Hematopoietic Stem Cell Transplantation
- The impact of COVID-19 on acute myeloid leukemia patients undergoing allogeneic stem cell transplantation: a concise review
- A Case Report of the Second de Novo Acute Myeloid Leukemia (AML) Following Allogeneic Stem Cell Transplantation in a Patient with the First AML
- Two Cases of Generalized Vitiligo after Allogeneic Stem Cell Transplantation
- Pediatric Allogeneic Hematopoietic Stem Cell Transplantation in Korea: April 2000: The Korean Society of Pediatric Hematology-Oncology