Endocrinol Metab.
2021 Aug;36(4):745-756. 10.3803/EnM.2021.405.
Effects of Intermittent Fasting on the Circulating Levels and Circadian Rhythms of Hormones
- Affiliations
-
- 1Department of Biochemistry and Molecular Biology, Seoul National University College of Medicine, Seoul, Korea
- 2Genomic Medicine Institute, Medical Research Center, Seoul National University, Seoul, Korea
- 3Seoul National University College of Medicine, Seoul, Korea
- 4Division of Endocrinology and Metabolism, Department of Internal Medicine, Diabetes Center, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 5Department of Brain and Cognitive Sciences, Daegu Gyeongbuk Institute of Science and Technology (DGIST), Daegu, Korea
- 6Brown Institute of Molecular Medicine and Department of Neurobiology and Anatomy, McGovern Medical School of UTHealth, and MD Anderson Cancer Center & UTHealth Graduate School of Biomedical Sciences, Houston, TX, USA
- 7Department of Biomedical Sciences, Seoul National University College of Medicine, Seoul, Korea
- KMID: 2519662
- DOI: http://doi.org/10.3803/EnM.2021.405
Abstract
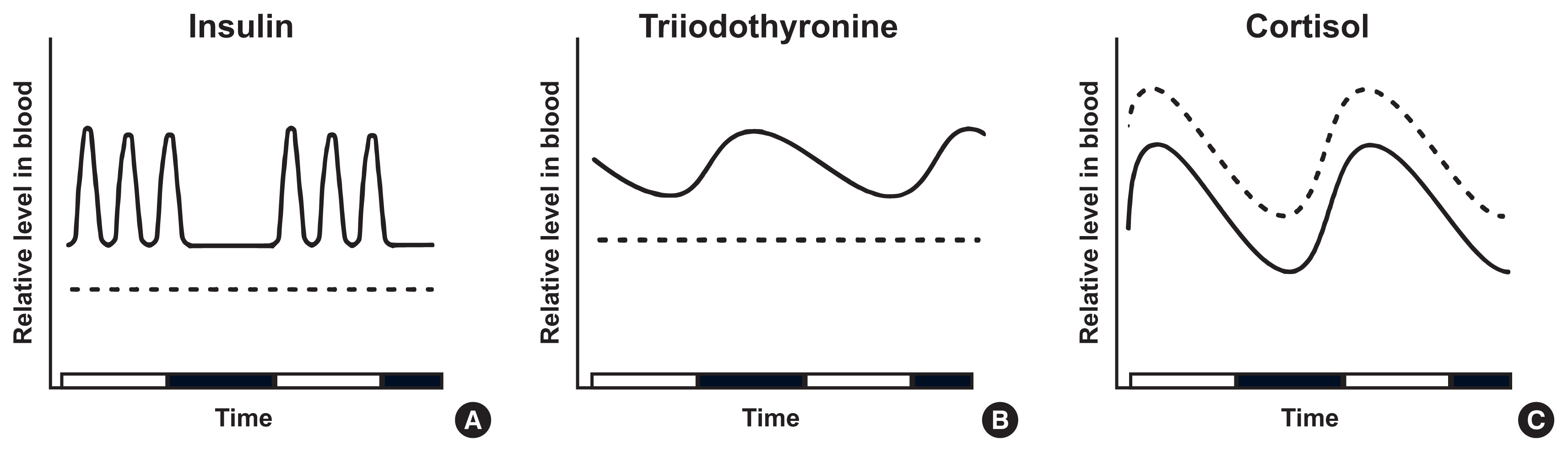
- Intermittent fasting has become an increasingly popular strategy in losing weight and associated reduction in obesity-related medical complications. Overwhelming studies support metabolic improvements from intermittent fasting in blood glucose levels, cardiac and brain function, and other health benefits, in addition to weight loss. However, concerns have also been raised on side effects including muscle loss, ketosis, and electrolyte imbalance. Of particular concern, the effect of intermittent fasting on hormonal circadian rhythms has received little attention. Given the known importance of circadian hormonal changes to normal physiology, potential detrimental effects by dysregulation of hormonal changes deserve careful discussions. In this review, we describe the changes in circadian rhythms of hormones caused by intermittent fasting. We covered major hormones commonly pathophysiologically involved in clinical endocrinology, including insulin, thyroid hormones, and glucocorticoids. Given that intermittent fasting could alter both the level and frequency of hormone secretion, decisions on practicing intermittent fasting should take more considerations on potential detrimental consequences versus beneficial effects pertaining to individual health conditions.
Figure
Cited by 1 articles
-
All That Glitters Is Not Gold: The Same Sleep Time, but Different Diabetogenic Outcomes
Bohye Kim, Obin Kwon
Endocrinol Metab. 2023;38(1):78-80. doi: 10.3803/EnM.2023.107.
Reference
-
1. Wyse CA, Biello SM, Gill JM. The bright-nights and dim-days of the urban photoperiod: implications for circadian rhythmicity, metabolism and obesity. Ann Med. 2014; 46:253–63.
Article2. Seaman DR. Weight gain as a consequence of living a modern lifestyle: a discussion of barriers to effective weight control and how to overcome them. J Chiropr Humanit. 2013; 20:27–35.
Article3. Patterson RE, Sears DD. Metabolic effects of intermittent fasting. Annu Rev Nutr. 2017; 37:371–93.
Article4. Rynders CA, Thomas EA, Zaman A, Pan Z, Catenacci VA, Melanson EL. Effectiveness of intermittent fasting and time-restricted feeding compared to continuous energy restriction for weight loss. Nutrients. 2019; 11:2442.
Article5. Harvie M, Howell A. Potential benefits and harms of intermittent energy restriction and intermittent fasting amongst obese, overweight and normal weight subjects: a narrative review of human and animal evidence. Behav Sci (Basel). 2017; 7:4.
Article6. Johnson JB, Summer W, Cutler RG, Martin B, Hyun DH, Dixit VD, et al. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic Biol Med. 2007; 42:665–74.
Article7. Harvie MN, Pegington M, Mattson MP, Frystyk J, Dillon B, Evans G, et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int J Obes (Lond). 2011; 35:714–27.
Article8. Cignarella F, Cantoni C, Ghezzi L, Salter A, Dorsett Y, Chen L, et al. Intermittent fasting confers protection in CNS autoimmunity by altering the gut microbiota. Cell Metab. 2018; 27:1222–35.
Article9. Singh R, Lakhanpal D, Kumar S, Sharma S, Kataria H, Kaur M, et al. Late-onset intermittent fasting dietary restriction as a potential intervention to retard age-associated brain function impairments in male rats. Age (Dordr). 2012; 34:917–33.
Article10. Fontan-Lozano A, Saez-Cassanelli JL, Inda MC, de los Santos-Arteaga M, Sierra-Dominguez SA, Lopez-Lluch G, et al. Caloric restriction increases learning consolidation and facilitates synaptic plasticity through mechanisms dependent on NR2B subunits of the NMDA receptor. J Neurosci. 2007; 27:10185–95.
Article11. Longo VD, Panda S. Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metab. 2016; 23:1048–59.
Article12. Mattson MP, de Cabo R. Effects of intermittent fasting on health, aging, and disease: reply. N Engl J Med. 2020; 382:1773–4.13. Tinsley GM, La Bounty PM. Effects of intermittent fasting on body composition and clinical health markers in humans. Nutr Rev. 2015; 73:661–74.
Article14. Lowe DA, Wu N, Rohdin-Bibby L, Moore AH, Kelly N, Liu YE, et al. Effects of time-restricted eating on weight loss and other metabolic parameters in women and men with overweight and obesity: the TREAT randomized clinical trial. JAMA Intern Med. 2020; 180:1491–9.
Article15. Soeters MR, Lammers NM, Dubbelhuis PF, Ackermans M, Jonkers-Schuitema CF, Fliers E, et al. Intermittent fasting does not affect whole-body glucose, lipid, or protein metabolism. Am J Clin Nutr. 2009; 90:1244–51.
Article16. Attarzadeh Hosseini SR, Sardar MA, Hejazi K, Farahati S. The effect of Ramadan fasting and physical activity on body composition, serum osmolarity levels and some parameters of electrolytes in females. Int J Endocrinol Metab. 2013; 11:88–94.
Article17. Munoz-Hernandez L, Marquez-Lopez Z, Mehta R, Aguilar-Salinas CA. Intermittent fasting as part of the management for T2DM: from animal models to human clinical studies. Curr Diab Rep. 2020; 20:13.
Article18. Mrosovsky N, Reebs SG, Honrado GI, Salmon PA. Behavioural entrainment of circadian rhythms. Experientia. 1989; 45:696–702.
Article19. Pittendrigh CS. Temporal organization: reflections of a Darwinian clock-watcher. Annu Rev Physiol. 1993; 55:16–54.
Article20. Hastings MH, Maywood ES, Brancaccio M. Generation of circadian rhythms in the suprachiasmatic nucleus. Nat Rev Neurosci. 2018; 19:453–69.
Article21. Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002; 418:935–41.
Article22. Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2012; 35:445–62.
Article23. Panda S. Circadian physiology of metabolism. Science. 2016; 354:1008–15.
Article24. Lee JH, Verma N, Thakkar N, Yeung C, Sung HK. Intermittent fasting: physiological implications on outcomes in mice and men. Physiology (Bethesda). 2020; 35:185–95.
Article25. Patterson RE, Laughlin GA, LaCroix AZ, Hartman SJ, Natarajan L, Senger CM, et al. Intermittent fasting and human metabolic health. J Acad Nutr Diet. 2015; 115:1203–12.
Article26. Wilcox G. Insulin and insulin resistance. Clin Biochem Rev. 2005; 26:19–39.27. Petersen MC, Shulman GI. Mechanisms of insulin action and insulin resistance. Physiol Rev. 2018; 98:2133–223.
Article28. Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, Ko CH, et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010; 466:627–31.
Article29. Perelis M, Marcheva B, Ramsey KM, Schipma MJ, Hutchison AL, Taguchi A, et al. Pancreatic β cell enhancers regulate rhythmic transcription of genes controlling insulin secretion. Science. 2015; 350:aac4250.
Article30. Vieira E, Burris TP, Quesada I. Clock genes, pancreatic function, and diabetes. Trends Mol Med. 2014; 20:685–93.
Article31. Qian J, Block GD, Colwell CS, Matveyenko AV. Consequences of exposure to light at night on the pancreatic islet circadian clock and function in rats. Diabetes. 2013; 62:3469–78.
Article32. Buijs RM, Chun SJ, Niijima A, Romijn HJ, Nagai K. Parasympathetic and sympathetic control of the pancreas: a role for the suprachiasmatic nucleus and other hypothalamic centers that are involved in the regulation of food intake. J Comp Neurol. 2001; 431:405–23.
Article33. Nyholm B, Walker M, Gravholt CH, Shearing PA, Sturis J, Alberti KG, et al. Twenty-four-hour insulin secretion rates, circulating concentrations of fuel substrates and gut incretin hormones in healthy offspring of type II (non-insulin-dependent) diabetic parents: evidence of several aberrations. Diabetologia. 1999; 42:1314–23.
Article34. Polonsky KS, Given BD, Van Cauter E. Twenty-four-hour profiles and pulsatile patterns of insulin secretion in normal and obese subjects. J Clin Invest. 1988; 81:442–8.
Article35. Ahren B. Diurnal variation in circulating leptin is dependent on gender, food intake and circulating insulin in mice. Acta Physiol Scand. 2000; 169:325–31.
Article36. Stenvers DJ, Scheer FAJL, Schrauwen P, la Fleur SE, Kalsbeek A. Circadian clocks and insulin resistance. Nat Rev Endocrinol. 2019; 15:75–89.
Article37. Dyar KA, Ciciliot S, Wright LE, Bienso RS, Tagliazucchi GM, Patel VR, et al. Muscle insulin sensitivity and glucose metabolism are controlled by the intrinsic muscle clock. Mol Metab. 2013; 3:29–41.
Article38. Gliniak CM, Brown JM, Noy N. The retinol-binding protein receptor STRA6 regulates diurnal insulin responses. J Biol Chem. 2017; 292:15080–93.
Article39. Duan W, Guo Z, Jiang H, Ware M, Mattson MP. Reversal of behavioral and metabolic abnormalities, and insulin resistance syndrome, by dietary restriction in mice deficient in brain-derived neurotrophic factor. Endocrinology. 2003; 144:2446–53.
Article40. Anson RM, Guo Z, de Cabo R, Iyun T, Rios M, Hagepanos A, et al. Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc Natl Acad Sci U S A. 2003; 100:6216–20.
Article41. Rubin NH, Alinder G, Rietveld WJ, Rayford PL, Thompson JC. Restricted feeding schedules alter the circadian rhythms of serum insulin and gastric inhibitory polypeptide. Regul Pept. 1988; 23:279–88.
Article42. Klein S, Sakurai Y, Romijn JA, Carroll RM. Progressive alterations in lipid and glucose metabolism during short-term fasting in young adult men. Am J Physiol. 1993; 265(5 Pt 1):E801–6.
Article43. Heilbronn LK, Smith SR, Martin CK, Anton SD, Ravussin E. Alternate-day fasting in nonobese subjects: effects on body weight, body composition, and energy metabolism. Am J Clin Nutr. 2005; 81:69–73.
Article44. Harvie M, Wright C, Pegington M, McMullan D, Mitchell E, Martin B, et al. The effect of intermittent energy and carbohydrate restriction v. daily energy restriction on weight loss and metabolic disease risk markers in overweight women. Br J Nutr. 2013; 110:1534–47.
Article45. Cienfuegos S, Gabel K, Kalam F, Ezpeleta M, Wiseman E, Pavlou V, et al. Effects of 4- and 6-h time-restricted feeding on weight and cardiometabolic health: a randomized controlled trial in adults with obesity. Cell Metab. 2020; 32:366–78.
Article46. Ikegami K, Refetoff S, Van Cauter E, Yoshimura T. Interconnection between circadian clocks and thyroid function. Nat Rev Endocrinol. 2019; 15:590–600.
Article47. Mullur R, Liu YY, Brent GA. Thyroid hormone regulation of metabolism. Physiol Rev. 2014; 94:355–82.
Article48. Fekete C, Lechan RM. Central regulation of hypothalamic-pituitary-thyroid axis under physiological and pathophysiological conditions. Endocr Rev. 2014; 35:159–94.
Article49. Kalsbeek A, Fliers E, Franke AN, Wortel J, Buijs RM. Functional connections between the suprachiasmatic nucleus and the thyroid gland as revealed by lesioning and viral tracing techniques in the rat. Endocrinology. 2000; 141:3832–41.
Article50. Aninye IO, Matsumoto S, Sidhaye AR, Wondisford FE. Circadian regulation of Tshb gene expression by Rev-Erbα (NR1D1) and nuclear corepressor 1 (NCOR1). J Biol Chem. 2014; 289:17070–7.
Article51. Jordan D, Rousset B, Perrin F, Fournier M, Orgiazzi J. Evidence for circadian variations in serum thyrotropin, 3,5, 3′-triiodothyronine, and thyroxine in the rat. Endocrinology. 1980; 107:1245–8.
Article52. Philippe J, Dibner C. Thyroid circadian timing: roles in physiology and thyroid malignancies. J Biol Rhythms. 2015; 30:76–83.53. Russell W, Harrison RF, Smith N, Darzy K, Shalet S, Weetman AP, et al. Free triiodothyronine has a distinct circadian rhythm that is delayed but parallels thyrotropin levels. J Clin Endocrinol Metab. 2008; 93:2300–6.
Article54. de Vries EM, van Beeren HC, van Wijk AC, Kalsbeek A, Romijn JA, Fliers E, et al. Regulation of type 3 deiodinase in rodent liver and adipose tissue during fasting. Endocr Connect. 2020; 9:552–62.
Article55. Galton VA, Hernandez A, St Germain DL. The 5′-deiodinases are not essential for the fasting-induced decrease in circulating thyroid hormone levels in male mice: possible roles for the type 3 deiodinase and tissue sequestration of hormone. Endocrinology. 2014; 155:3172–81.
Article56. Boelen A, Wiersinga WM, Fliers E. Fasting-induced changes in the hypothalamus-pituitary-thyroid axis. Thyroid. 2008; 18:123–9.
Article57. Legradi G, Emerson CH, Ahima RS, Flier JS, Lechan RM. Leptin prevents fasting-induced suppression of prothyrotropin-releasing hormone messenger ribonucleic acid in neurons of the hypothalamic paraventricular nucleus. Endocrinology. 1997; 138:2569–76.
Article58. Lechan RM, Fekete C. Role of melanocortin signaling in the regulation of the hypothalamic-pituitary-thyroid (HPT) axis. Peptides. 2006; 27:310–25.
Article59. Guo F, Bakal K, Minokoshi Y, Hollenberg AN. Leptin signaling targets the thyrotropin-releasing hormone gene promoter in vivo. Endocrinology. 2004; 145:2221–7.
Article60. Ortiga-Carvalho TM, Curty FH, Nascimento-Saba CC, Moura EG, Polak J, Pazos-Moura CC. Pituitary neuromedin B content in experimental fasting and diabetes mellitus and correlation with thyrotropin secretion. Metabolism. 1997; 46:149–53.
Article61. Gardner DF, Kaplan MM, Stanley CA, Utiger RD. Effect of tri-iodothyronine replacement on the metabolic and pituitary responses to starvation. N Engl J Med. 1979; 300:579–84.
Article62. Merimee TJ, Fineberg ES. Starvation-induced alterations of circulating thyroid hormone concentrations in man. Metabolism. 1976; 25:79–83.
Article63. Stekovic S, Hofer SJ, Tripolt N, Aon MA, Royer P, Pein L, et al. Alternate day fasting improves physiological and molecular markers of aging in healthy, non-obese humans. Cell Metab. 2019; 30:462–76.
Article64. Moro T, Tinsley G, Bianco A, Marcolin G, Pacelli QF, Battaglia G, et al. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J Transl Med. 2016; 14:290.
Article65. Chung S, Son GH, Kim K. Circadian rhythm of adrenal glucocorticoid: its regulation and clinical implications. Biochim Biophys Acta. 2011; 1812:581–91.
Article66. Nicolaides NC, Charmandari E, Chrousos GP, Kino T. Circadian endocrine rhythms: the hypothalamic-pituitary-adrenal axis and its actions. Ann N Y Acad Sci. 2014; 1318:71–80.
Article67. Kim ER, Xu Y, Cassidy RM, Lu Y, Yang Y, Tian J, et al. Paraventricular hypothalamus mediates diurnal rhythm of metabolism. Nat Commun. 2020; 11:3794.
Article68. Zhu C, Xu Y, Jiang Z, Tian JB, Cassidy RM, Cai ZL, et al. Disrupted hypothalamic CRH neuron responsiveness contributes to diet-induced obesity. EMBO Rep. 2020; 21:e49210.69. Buijs RM, Kalsbeek A. Hypothalamic integration of central and peripheral clocks. Nat Rev Neurosci. 2001; 2:521–6.
Article70. Ulrich-Lai YM, Arnhold MM, Engeland WC. Adrenal splanchnic innervation contributes to the diurnal rhythm of plasma corticosterone in rats by modulating adrenal sensitivity to ACTH. Am J Physiol Regul Integr Comp Physiol. 2006; 290:R1128–35.
Article71. Son GH, Chung S, Choe HK, Kim HD, Baik SM, Lee H, et al. Adrenal peripheral clock controls the autonomous circadian rhythm of glucocorticoid by causing rhythmic steroid production. Proc Natl Acad Sci U S A. 2008; 105:20970–5.
Article72. Kakihana R, Moore JA. Circadian rhythm of corticosterone in mice: the effect of chronic consumption of alcohol. Psychopharmacologia. 1976; 46:301–5.
Article73. Dickmeis T. Glucocorticoids and the circadian clock. J Endocrinol. 2009; 200:3–22.
Article74. Morris CJ, Aeschbach D, Scheer FA. Circadian system, sleep and endocrinology. Mol Cell Endocrinol. 2012; 349:91–104.
Article75. Bellinger LL, Mendel VE, Moberg GP. Circadian insulin, GH, prolactin, corticosterone and glucose rhythms in fed and fasted rats. Horm Metab Res. 1975; 7:132–5.
Article76. Morimoto Y, Arisue K, Yamamura Y. Relationship between circadian rhythm of food intake and that of plasma corticosterone and effect of food restriction on circadian adrenocortical rhythm in the rat. Neuroendocrinology. 1977; 23:212–22.
Article77. Wilkinson CW, Shinsako J, Dallman MF. Daily rhythms in adrenal responsiveness to adrenocorticotropin are determined primarily by the time of feeding in the rat. Endocrinology. 1979; 104:350–9.
Article78. Hojlund K, Wildner-Christensen M, Eshoj O, Skjaerbaek C, Holst JJ, Koldkjaer O, et al. Reference intervals for glucose, beta-cell polypeptides, and counterregulatory factors during prolonged fasting. Am J Physiol Endocrinol Metab. 2001; 280:E50–8.79. Bergendahl M, Vance ML, Iranmanesh A, Thorner MO, Veldhuis JD. Fasting as a metabolic stress paradigm selectively amplifies cortisol secretory burst mass and delays the time of maximal nyctohemeral cortisol concentrations in healthy men. J Clin Endocrinol Metab. 1996; 81:692–9.
Article80. Johnstone AM, Faber P, Andrew R, Gibney ER, Elia M, Lobley G, et al. Influence of short-term dietary weight loss on cortisol secretion and metabolism in obese men. Eur J Endocrinol. 2004; 150:185–94.
Article81. Schurgin S, Canavan B, Koutkia P, Depaoli AM, Grinspoon S. Endocrine and metabolic effects of physiologic r-metHuLeptin administration during acute caloric deprivation in normal-weight women. J Clin Endocrinol Metab. 2004; 89:5402–9.
Article82. Veldhuis JD, Iranmanesh A, Evans WS, Lizarralde G, Thorner MO, Vance ML. Amplitude suppression of the pulsatile mode of immunoradiometric luteinizing hormone release in fasting-induced hypoandrogenemia in normal men. J Clin Endocrinol Metab. 1993; 76:587–93.
Article83. Jamshed H, Beyl RA, Della Manna DL, Yang ES, Ravussin E, Peterson CM. Early time-restricted feeding improves 24-hour glucose levels and affects markers of the circadian clock, aging, and autophagy in humans. Nutrients. 2019; 11:1234.
Article84. Lu M, Flanagan JU, Langley RJ, Hay MP, Perry JK. Targeting growth hormone function: strategies and therapeutic applications. Signal Transduct Target Ther. 2019; 4:3.
Article85. Yulyaningsih E, Loh K, Lin S, Lau J, Zhang L, Shi Y, et al. Pancreatic polypeptide controls energy homeostasis via Npy6r signaling in the suprachiasmatic nucleus in mice. Cell Metab. 2014; 19:58–72.
Article86. Steyn FJ, Huang L, Ngo ST, Leong JW, Tan HY, Xie TY, et al. Development of a method for the determination of pulsatile growth hormone secretion in mice. Endocrinology. 2011; 152:3165–71.
Article87. Bednarz K, Alshafie W, Aufmkolk S, Desserteaux T, Markam PS, Storch KF, et al. Ultradian secretion of growth hormone in mice: linking physiology with changes in synapse parameters using super-resolution microscopy. Front Neural Circuits. 2020; 14:21.
Article88. Takahashi Y, Kipnis DM, Daughaday WH. Growth hormone secretion during sleep. J Clin Invest. 1968; 47:2079–90.
Article89. Brandenberger G, Weibel L. The 24-h growth hormone rhythm in men: sleep and circadian influences questioned. J Sleep Res. 2004; 13:251–5.
Article90. Bruno JF, Olchovsky D, White JD, Leidy JW, Song J, Berelowitz M. Influence of food deprivation in the rat on hypothalamic expression of growth hormone-releasing factor and somatostatin. Endocrinology. 1990; 127:2111–6.
Article91. Huang L, Tan HY, Fogarty MJ, Andrews ZB, Veldhuis JD, Herzog H, et al. Actions of NPY, and its Y1 and Y2 receptors on pulsatile growth hormone secretion during the fed and fasted state. J Neurosci. 2014; 34:16309–19.
Article92. Moller L, Dalman L, Norrelund H, Billestrup N, Frystyk J, Moller N, et al. Impact of fasting on growth hormone signaling and action in muscle and fat. J Clin Endocrinol Metab. 2009; 94:965–72.
Article93. Ho KY, Veldhuis JD, Johnson ML, Furlanetto R, Evans WS, Alberti KG, et al. Fasting enhances growth hormone secretion and amplifies the complex rhythms of growth hormone secretion in man. J Clin Invest. 1988; 81:968–75.
Article94. Hartman ML, Veldhuis JD, Johnson ML, Lee MM, Alberti KG, Samojlik E, et al. Augmented growth hormone (GH) secretory burst frequency and amplitude mediate enhanced GH secretion during a two-day fast in normal men. J Clin Endocrinol Metab. 1992; 74:757–65.95. Avram AM, Jaffe CA, Symons KV, Barkan AL. Endogenous circulating ghrelin does not mediate growth hormone rhythmicity or response to fasting. J Clin Endocrinol Metab. 2005; 90:2982–7.
Article96. Buffenstein R, Poppitt SD, McDevitt RM, Prentice AM. Food intake and the menstrual cycle: a retrospective analysis, with implications for appetite research. Physiol Behav. 1995; 58:1067–77.
Article97. Miller BH, Takahashi JS. Central circadian control of female reproductive function. Front Endocrinol (Lausanne). 2014; 4:195.
Article98. Kumar S, Kaur G. Intermittent fasting dietary restriction regimen negatively influences reproduction in young rats: a study of hypothalamo-hypophysial-gonadal axis. PLoS One. 2013; 8:e52416.
Article99. Martin B, Pearson M, Kebejian L, Golden E, Keselman A, Bender M, et al. Sex-dependent metabolic, neuroendocrine, and cognitive responses to dietary energy restriction and excess. Endocrinology. 2007; 148:4318–33.
Article100. Hua L, Feng B, Huang L, Li J, Luo T, Jiang X, et al. Time-restricted feeding improves the reproductive function of female mice via liver fibroblast growth factor 21. Clin Transl Med. 2020; 10:e195.
Article101. Brzezinski A. Melatonin in humans. N Engl J Med. 1997; 336:186–95.
Article102. Uchida K, Okamoto N, Ohara K, Morita Y. Daily rhythm of serum melatonin in patients with dementia of the degenerate type. Brain Res. 1996; 717:154–9.
Article103. Selmaoui B, Touitou Y. Reproducibility of the circadian rhythms of serum cortisol and melatonin in healthy subjects: a study of three different 24-h cycles over six weeks. Life Sci. 2003; 73:3339–49.
Article104. Berga SL, Loucks TL, Cameron JL. Endocrine and chronobiological effects of fasting in women. Fertil Steril. 2001; 75:926–32.
Article105. Almeneessier AS, Bahammam AS, Sharif MM, Bahammam SA, Nashwan SZ, Pandi Perumal SR, et al. The influence of intermittent fasting on the circadian pattern of melatonin while controlling for caloric intake, energy expenditure, light exposure, and sleep schedules: a preliminary report. Ann Thorac Med. 2017; 12:183–90.
Article106. Mohammad-Zadeh LF, Moses L, Gwaltney-Brant SM. Serotonin: a review. J Vet Pharmacol Ther. 2008; 31:187–99.
Article107. Sauerbier I, von Mayersbach H. Circadian variation of serotonin levels in human blood. Horm Metab Res. 1976; 8:157–8.
Article108. Kwon O, Yu JH, Jeong E, Yoo HJ, Kim MS. Meal-related oscillations in the serum serotonin levels in healthy young men. Clin Endocrinol (Oxf). 2018; 88:549–55.
Article109. Sundar IK, Yao H, Huang Y, Lyda E, Sime PJ, Sellix MT, et al. Serotonin and corticosterone rhythms in mice exposed to cigarette smoke and in patients with COPD: implication for COPD-associated neuropathogenesis. PLoS One. 2014; 9:e87999.
Article110. Valdes-Fuentes M, Vera-Rivera G, De Ita-Perez D, Mendez I, Miranda MI, Diaz-Munoz M. Effect of daytime-restricted feeding in the daily variations of liver metabolism and blood transport of serotonin in rat. Physiol Rep. 2015; 3:e12389.
Article111. Hida K, Wada J, Eguchi J, Zhang H, Baba M, Seida A, et al. Visceral adipose tissue-derived serine protease inhibitor: a unique insulin-sensitizing adipocytokine in obesity. Proc Natl Acad Sci U S A. 2005; 102:10610–5.
Article112. Jeong E, Youn BS, Kim DW, Kim EH, Park JW, Namkoong C, et al. Circadian rhythm of serum vaspin in healthy male volunteers: relation to meals. J Clin Endocrinol Metab. 2010; 95:1869–75.
Article113. Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 2018; 27:1212–21.
Article114. Corley BT, Carroll RW, Hall RM, Weatherall M, Parry-Strong A, Krebs JD. Intermittent fasting in type 2 diabetes mellitus and the risk of hypoglycaemia: a randomized controlled trial. Diabet Med. 2018; 35:588–94.
Article115. Beta-blockers. Part II: the effect of associated disease states on the choice of a beta-blocker. Aust Nurses J. 1982; 11:31. 94.116. Ahmed SH, Chowdhury TA, Hussain S, Syed A, Karamat A, Helmy A, et al. Ramadan and diabetes: a narrative review and practice update. Diabetes Ther. 2020; 11:2477–520.
Article117. Akasheh RT, Kroeger CM, Trepanowski JF, Gabel K, Hoddy KK, Kalam F, et al. Weight loss efficacy of alternate day fasting versus daily calorie restriction in subjects with subclinical hypothyroidism: a secondary analysis. Appl Physiol Nutr Metab. 2020; 45:340–3.
Article118. Sheikh A, Mawani M, Mahar SA. Impact of Ramadan fasting on thyroid status and quality of life in patients with primary hypothyroidism: a prospective cohort study from Karachi, Pakistan. Endocr Pract. 2018; 24:882–8.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Measurement of Circadian Rhythms in the Research of Psychiatric Disorders
- Human Circadian Rhythms
- Cell Autonomous Circadian Systems and Their Relation to Inflammation
- Managing Circadian Rhythms: A Key to Enhancing Mental Health in College Students
- Relationship of Circadian Rhythm in Behavioral Characteristics and Lipid Peroxidation of Brain Tissues in Mice