Korean J Physiol Pharmacol.
2021 Sep;25(5):403-411. 10.4196/kjpp.2021.25.5.403.
Gastroprotective effect of cirsilineol against hydrochloric acid/ ethanol-induced gastric ulcer in rats
- Affiliations
-
- 1Department of Gastrointestinal Surgery, Xichang People's Hospital, Xichang 615000, China.
- 2Department of Gastroenterology, The Affiliated Hospital of Inner Mongolia University for Nationalities, Tongliao 028000, China.
- 3Department of Pharmacy, The Second Hospital of Jilin University, Changchun 130041, China.
- 4General Medicine Yantaishan Hospital, Yantai 264001, China.
- 5The People's Hospital of Guangxi Zhuang Autonomous Region, Nanning 530021, China.
- 6Chongqing University Affiliated Tumor Hospital/Chongqing Cancer Institute, Chongqing 400030, China.
- 7Department of Oncology, Shaanxi Forest Industry Hospital, Xi'an 710000, China.
- 8Department of Liver, Spleen and Stomach Diseases, First Affiliated Hospital of Heilongjiang University of Traditional Chinese Medicine, Heilongjiang 150040, China.
- KMID: 2519403
- DOI: http://doi.org/10.4196/kjpp.2021.25.5.403
Abstract
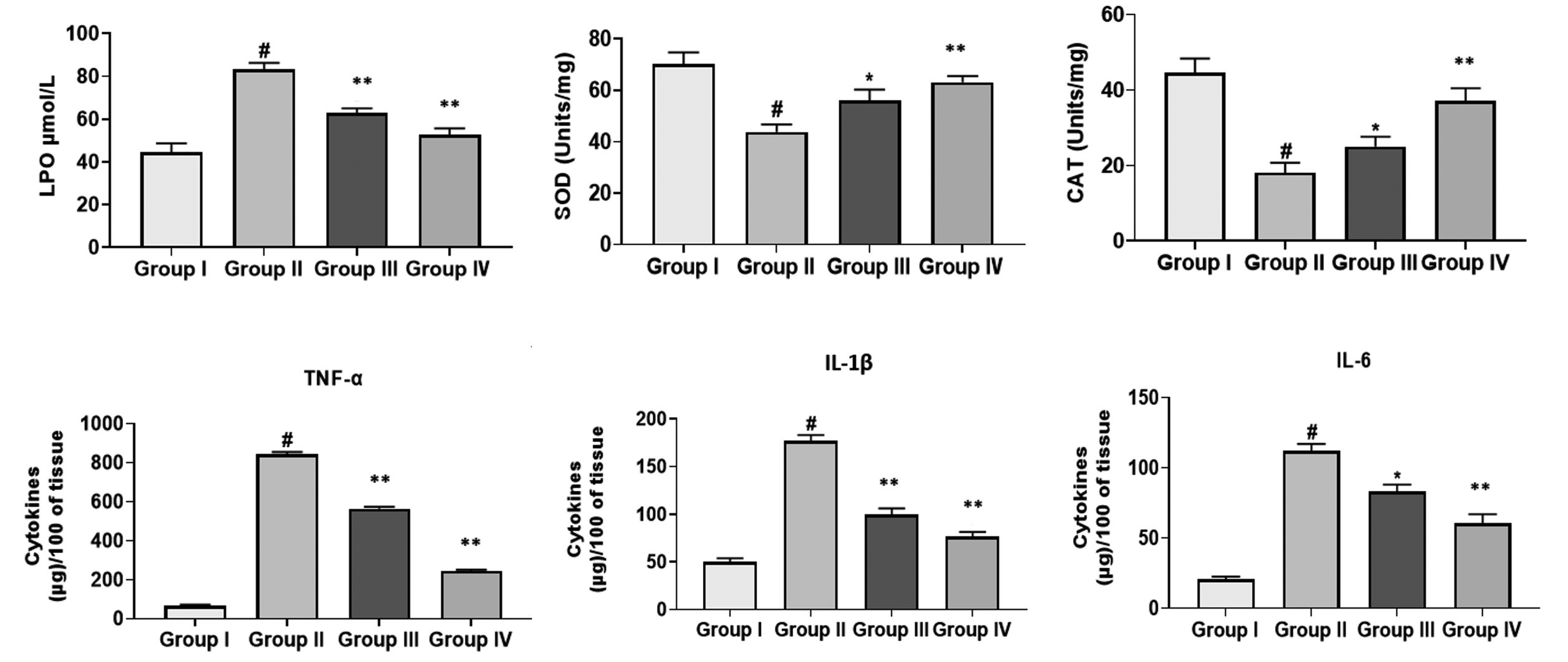
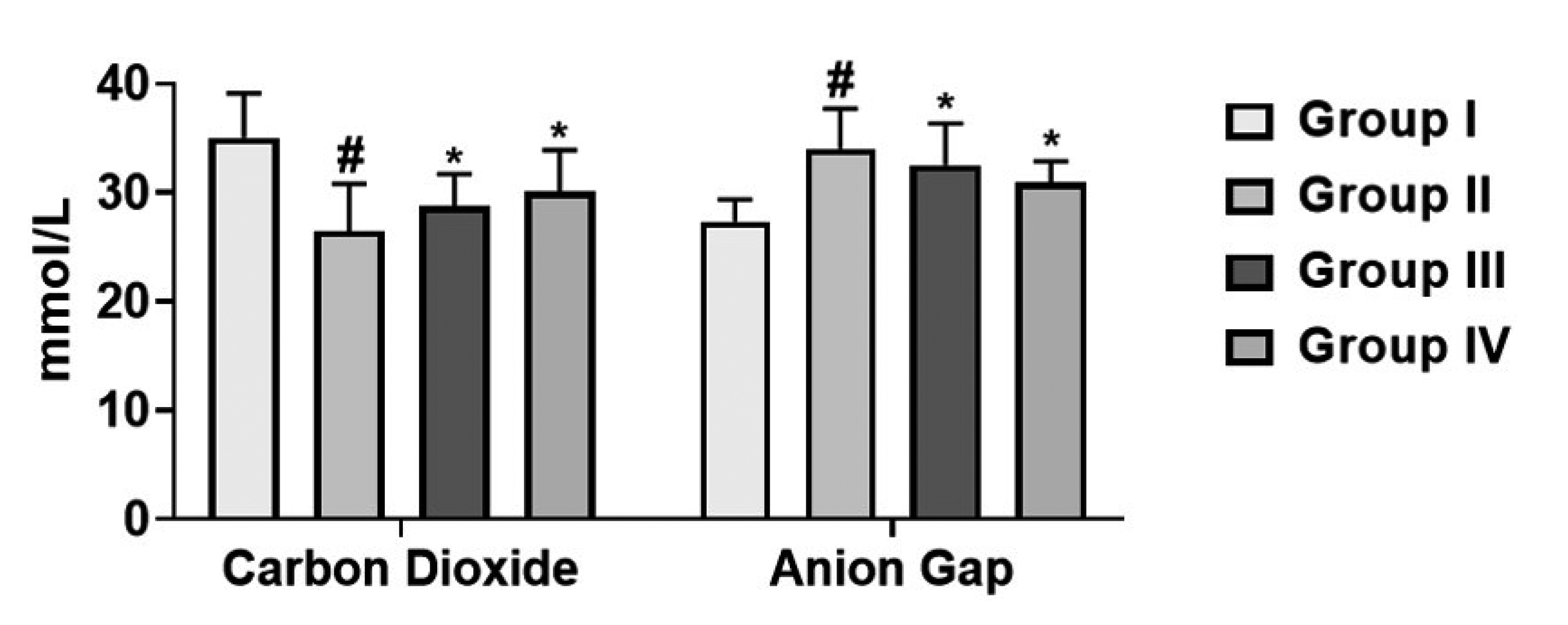
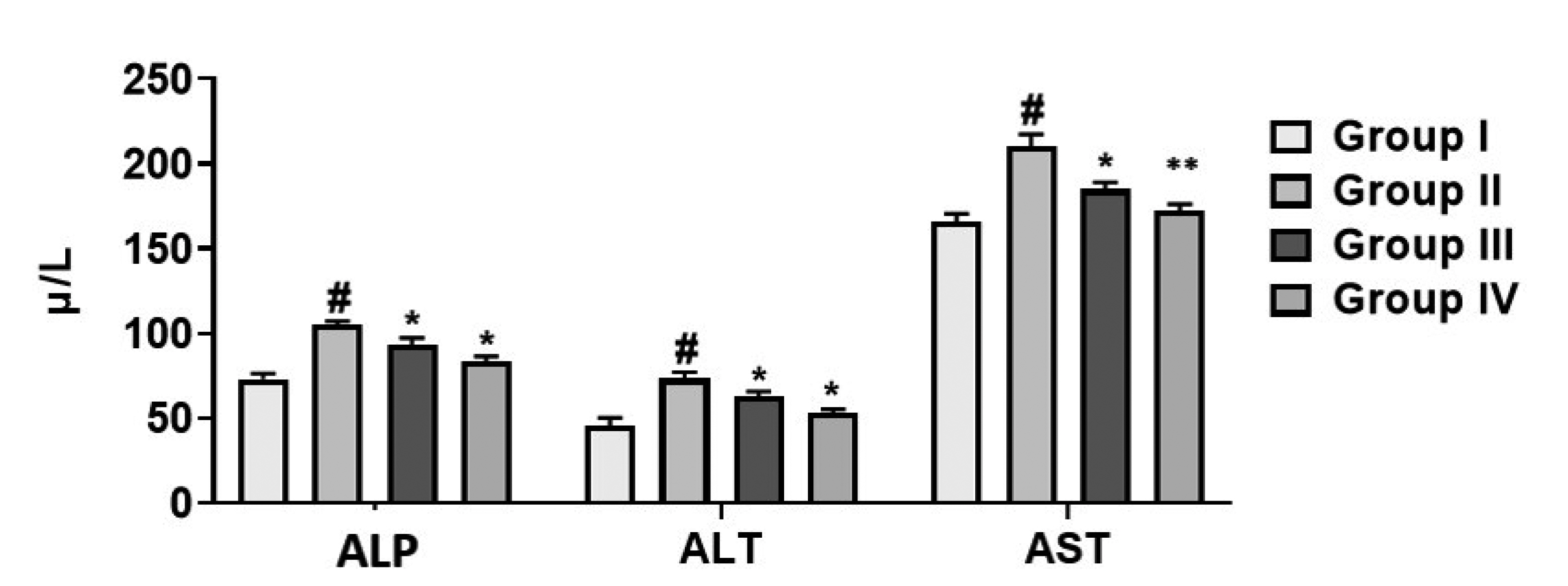
- This study was designed to evaluate the gastroprotective activity of cirsilineol in hydrochloric acid (HCl)/ethanol-induced gastric ulcer model. Cirsilineol was administered at the doses of 20 and 40 mg/kg in HCl/ethanol-induced rats. The gastroprotective ability was verified by determining the ulcer score, total acidity, hemoglobin, inflammatory cytokines, lipid peroxides, and enzymatic antioxidants superoxide dismutase (SOD) and catalase (CAT) in gastric tissue and serum biochemical analysis. The results showed a favorable increase in the hemoglobin level, antioxidant enzymes (SOD and CAT), restored electrochemical balance (carbon dioxide & anion gap) while a noticeable decrease in ulcer index, total acidity, lipid peroxides, inflammatory cytokines (interleukin-1 beta [IL-1β], IL-6, and tumor necrosis factor alpha) in rats treated with the cirsilineol. The serum biochemical analysis on liver markers (alkaline phosphatases, alanine aminotransferase, and aspartate aminotransferase), kidney markers (urea, creatinine, albumin, globulin, total protein), and lipid profile (triglyceride, high-density lipoprotein, total cholesterol) were attenuated by cirsilineol treatment in rats. Histopathology showed enhanced gastric protection and preserved the integrity of gastric mucosa upon cirsilineol administration. These results ultimately suggest that cirsilineol has gastroprotective effects that prevent the development of gastric ulcer.
Keyword
Figure
Cited by 1 articles
-
Experimental model and novel therapeutic targets for non-alcoholic fatty liver disease development
Yujin Jin, Kyung-Sun Heo
Korean J Physiol Pharmacol. 2023;27(4):299-310. doi: 10.4196/kjpp.2023.27.4.299.
Reference
-
1. Malfertheiner P, Chan FK, McColl KE. 2009; Peptic ulcer disease. Lancet. 374:1449–1461. DOI: 10.1016/S0140-6736(09)60938-7. PMID: 28242110.
Article2. Hamedi S, Arian AA, Farzaei MH. 2015; Gastroprotective effect of aqueous stem bark extract of Ziziphus jujuba L. against HCl/Ethanol-induced gastric mucosal injury in rats. J Tradit Chin Med. 35:666–670. DOI: 10.1016/S0254-6272(15)30157-6. PMID: 26742312.3. Sharifi-Rad M, Fokou PVT, Sharopov F, Martorell M, Ademiluyi AO, Rajkovic J, Salehi B, Martins N, Iriti M, Sharifi-Rad J. 2018; Antiulcer agents: from plant extracts to phytochemicals in healing promotion. Molecules. 23:1751. DOI: 10.3390/molecules23071751. PMID: 30018251. PMCID: PMC6100067.
Article4. Lin KJ, García Rodríguez LA, Hernández-Díaz S. 2011; Systematic review of peptic ulcer disease incidence rates: do studies without validation provide reliable estimates? Pharmacoepidemiol Drug Saf. 20:718–728. DOI: 10.1002/pds.2153. PMID: 21626606.
Article5. Wang AY, Peura DA. 2011; The prevalence and incidence of Helicobacter pylori-associated peptic ulcer disease and upper gastrointestinal bleeding throughout the world. Gastrointest Endosc Clin N Am. 21:613–635. DOI: 10.1016/j.giec.2011.07.011. PMID: 21944414.
Article6. Kangwan N, Park JM, Kim EH, Hahm KB. 2014; Quality of healing of gastric ulcers: natural products beyond acid suppression. World J Gastrointest Pathophysiol. 5:40–47. DOI: 10.4291/wjgp.v5.i1.40. PMID: 24891974. PMCID: PMC4024519.
Article7. Dacha S, Razvi M, Massaad J, Cai Q, Wehbi M. 2015; Hypergastrinemia. Gastroenterol Rep (Oxf). 3:201–208. DOI: 10.1093/gastro/gov004. PMID: 25698559. PMCID: PMC4527266.
Article8. Raza H, Abbas Q, Hassan M, Eo SH, Ashraf Z, Kim D, Phull AR, Kim SJ, Kang SK, Seo SY. 2017; Isolation, characterization, and in silico, in vitro and in vivo antiulcer studies of isoimperatorin crystallized from Ostericum koreanum. Pharm Biol. 55:218–226. DOI: 10.1080/13880209.2016.1257641. PMID: 27927061. PMCID: PMC6130598.
Article9. Mishra P, Mishra S. 2011; Study of antibacterial activity of Ocimum sanctum extract against gram positive and gram negative bacteria. Am J Food Technol. 6:336–341. DOI: 10.3923/ajft.2011.336.341.
Article10. Mondal S, Mahapatra SC, Mirdha BR, Naik SN. 2007; Antimicrobial activities of essential oils obtained from fresh and dried leaves of Ocimum sanctum (L.) against enteric bacteria and yeast. Acta Horticult. 756:267–269. DOI: 10.17660/ActaHortic.2007.756.28.11. Patil R, Patil R, Ahirwar B, Ahirwar D. 2011; Isolation and characterization of anti-diabetic component (bioactivity-guided fractionation) from Ocimum sanctum L. (Lamiaceae) aerial part. Asian Pac J Trop Med. 4:278–282. DOI: 10.1016/S1995-7645(11)60086-2. PMID: 21771470.12. Nagarajun S, Jain HC, Aulakh GS. Atal CK, Kapoor BM, editors. 1989. Indigenous plants used in fertility control. Cultivation and utilization of medicinal plants.13. Jin X, Liu Q, Dang M, Zhang W, Li X. 2019; Gastroprotective and ulcer healing properties of ethanol extract of Alpinia conchigera rhizome in experimentally induced gastric ulcer in rats. Trop J Pharm Res. 18:1873–1879. DOI: 10.4314/tjpr.v18i9.13.14. El-Maraghy SA, Rizk SM, Shahin NN. 2015; Gastroprotective effect of crocin in ethanol-induced gastric injury in rats. Chem Biol Interact. 229:26–35. DOI: 10.1016/j.cbi.2015.01.015. PMID: 25637687.
Article15. Yang Y, Yin B, Lv L, Wang Z, He J, Chen Z, Wen X, Zhang Y, Sun W, Li Y, Zhao Y. 2017; Gastroprotective effect of aucubin against ethanol-induced gastric mucosal injury in mice. Life Sci. 189:44–51. DOI: 10.1016/j.lfs.2017.09.016. PMID: 28919395.
Article16. Marklund S, Marklund G. 1974; Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 47:469–474. DOI: 10.1111/j.1432-1033.1974.tb03714.x. PMID: 4215654.
Article17. Aebi HE. Bergmeyer HU, editor. 1983. Catalase. Methods of enzymatic analysis. Verlag Chemie;Weinhem: p. 273–286.
Article18. da Silva LM, Boeing T, Somensi LB, Cury BJ, Steimbach VM, Silveria AC, Niero R, Cechinel Filho V, Santin JR, de Andrade SF. 2015; Evidence of gastric ulcer healing activity of Maytenus robusta Reissek: in vitro and in vivo studies. J Ethnopharmacol. 175:75–85. DOI: 10.1016/j.jep.2015.09.006. PMID: 26364940.19. Jelski W, Kozlowski M, Laudanski J, Niklinski J, Szmitkowski M. 2009; The activity of class I, II, III, and IV alcohol dehydrogenase (ADH) isoenzymes and aldehyde dehydrogenase (ALDH) in esophageal cancer. Dig Dis Sci. 54:725–730. DOI: 10.1007/s10620-008-0422-8. PMID: 18688716.
Article20. Zhou D, Yang Q, Tian T, Chang Y, Li Y, Duan LR, Li H, Wang SW. 2020; Gastroprotective effect of gallic acid against ethanol-induced gastric ulcer in rats: involvement of the Nrf2/HO-1 signaling and anti-apoptosis role. Biomed Pharmacother. 126:110075. DOI: 10.1016/j.biopha.2020.110075. PMID: 32179202.
Article21. Arab HH, Salama SA, Omar HA, Arafa el-SA, Maghrabi IA. 2015; Diosmin protects against ethanol-induced gastric injury in rats: novel anti-ulcer actions. PLoS One. 10:e0122417. DOI: 10.1371/journal.pone.0122417. PMID: 25821971. PMCID: PMC4378914.
Article22. Saranya P, Geetha A, Selvamathy SM. 2011; A biochemical study on the gastroprotective effect of andrographolide in rats induced with gastric ulcer. Indian J Pharm Sci. 73:550–557. DOI: 10.4103/0250-474X.99012. PMID: 22923868. PMCID: PMC3425067.
Article23. Mahendran P, Sabitha KE, Devi CS. 2002; Prevention of HCl-ethanol induced gastric mucosal injury in rats by Garcinia cambogia extract and its possible mechanism of action. Indian J Exp Biol. 40:58–62. PMID: 12561970.24. Abdelwahab SI. 2013; Protective mechanism of gallic acid and its novel derivative against ethanol-induced gastric ulcerogenesis: involvement of immunomodulation markers, Hsp70 and Bcl-2-associated X protein. Int Immunopharmacol. 16:296–305. DOI: 10.1016/j.intimp.2013.04.005. PMID: 23602913.
Article25. Song SH, Kim JE, Sung JE, Lee HA, Yun WB, Lee YH, Song H, Hwang D. 2018; Anti-ulcer effect of Gallarhois extract with anti-oxidant activity in an ICR model of ethanol/hydrochloride acid-induced gastric injury. J Tradit Complement Med. 9:372–382. DOI: 10.1016/j.jtcme.2017.07.001. PMID: 31453134. PMCID: PMC6701826.
Article26. Swarnakar S, Mishra A, Ganguly K, Sharma AV. 2007; Matrix metalloproteinase-9 activity and expression is reduced by melatonin during prevention of ethanol-induced gastric ulcer in mice. J Pineal Res. 43:56–64. DOI: 10.1111/j.1600-079X.2007.00443.x. PMID: 17614836.
Article27. Antonisamy P, Duraipandiyan V, Aravinthan A, Al-Dhabi NA, Ignacimuthu S, Choi KC, Kim JH. 2015; Protective effects of friedelin isolated from Azima tetracantha Lam. against ethanol-induced gastric ulcer in rats and possible underlying mechanisms. Eur J Pharmacol. 750:167–175. DOI: 10.1016/j.ejphar.2015.01.015. PMID: 25617794.
Article28. Kumari RP, Anbarasu K. 2014; Protective role of C-phycocyanin against secondary changes during sodium selenite mediated cataractogenesis. Nat Prod Bioprospect. 4:81–89. DOI: 10.1007/s13659-014-0008-4. PMID: 24858035. PMCID: PMC4004860.
Article29. Sidahmed HM, Hashim NM, Mohan S, Abdelwahab SI, Taha MM, Dehghan F, Yahayu M, Ee GC, Loke MF, Vadivelu J. 2016; Evidence of the gastroprotective and anti-Helicobacter pylori activities of β-mangostin isolated from Cratoxylum arborescens (vahl) blume. Drug Des Devel Ther. 10:297–313. DOI: 10.2147/DDDT.S80625. PMID: 26834460. PMCID: PMC4716727.30. Al-Wajeeh NS, Hajrezaie M, Al-Henhena N, Kamran S, Bagheri E, Zahedifard M, Saremi K, Noor SM, Ali HM, Abdulla MA. 2017; The antiulcer effect of Cibotium barometz leaves in rats with experimentally induced acute gastric ulcer. Drug Des Devel Ther. 11:995–1009. DOI: 10.2147/DDDT.S107018. PMID: 28408799. PMCID: PMC5384742.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Gastroprotective effects of irsogladine maleate on ethanol/hydrochloric acid induced gastric ulcers in mice
- Assessment of Antisecretory, Gastroprotective, and
In-vitro Antacid Potential ofDaucus carota in Experimental Rats - Gastroprotective Effects of Glutinous Rice Extract against Ethanol-, Indomethacin-, and Stress-induced Ulcers in Rats
- Gastroprotective Activities of Sennoside A and Sennoside B via the Up-Regulation of Prostaglandin E2 and the Inhibition of H+/K+-ATPase
- Comparison of the response using ICR mice derived from three different sources to ethanol/hydrochloric acid-induced gastric injury