Int J Stem Cells.
2021 Aug;14(3):341-350. 10.15283/ijsc21001.
MiR-181a Promotes Spermatogenesis by Targeting the S6K1 Pathway
- Affiliations
-
- 1Reproductive Medical Center, Zaozhuang Maternal and Child Health Hospital, Zaozhuang, China
- 2Department of Gynaecology, Zaozhuang Maternal and Child Health Hospital, Zaozhuang, China
- 3Reproductive Medical Center, Qingdao Women and Children’s Hospital, Qingdao University, Qingdao, China
- KMID: 2519376
- DOI: http://doi.org/10.15283/ijsc21001
Abstract
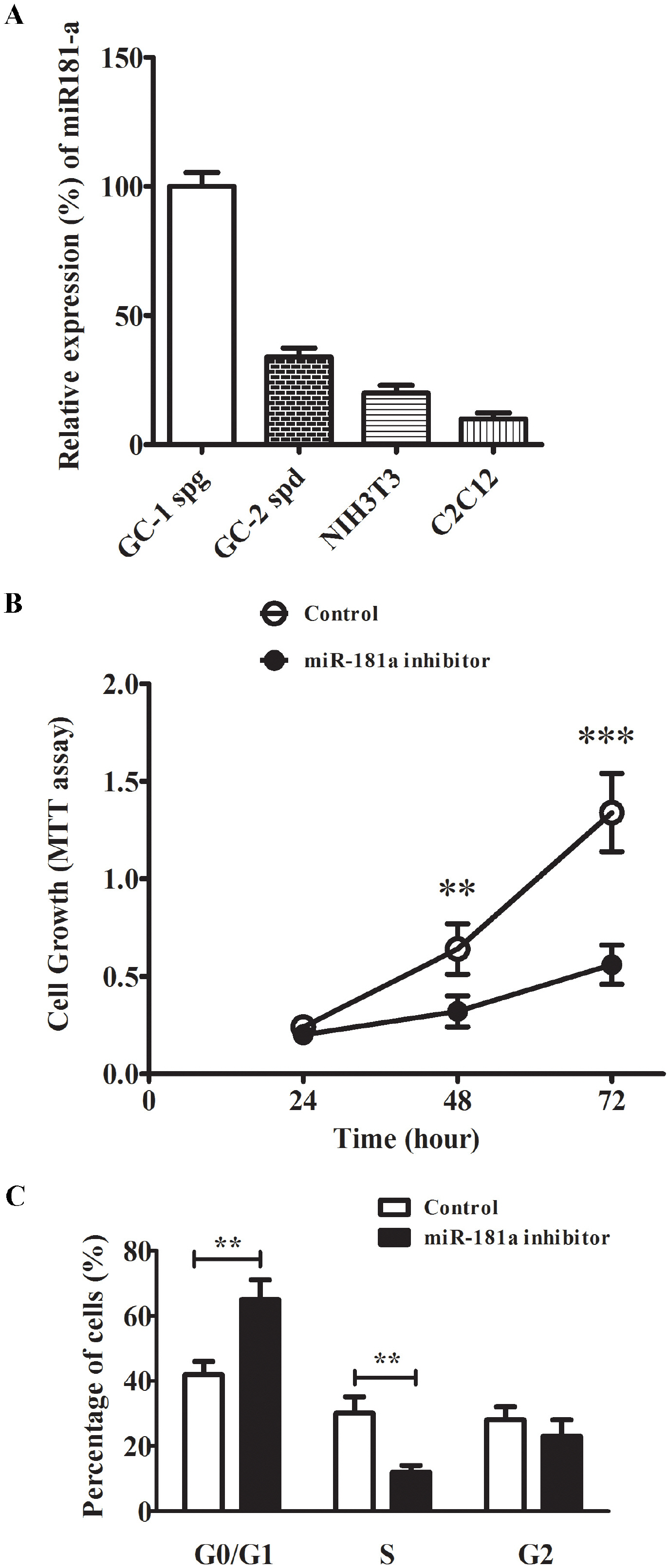
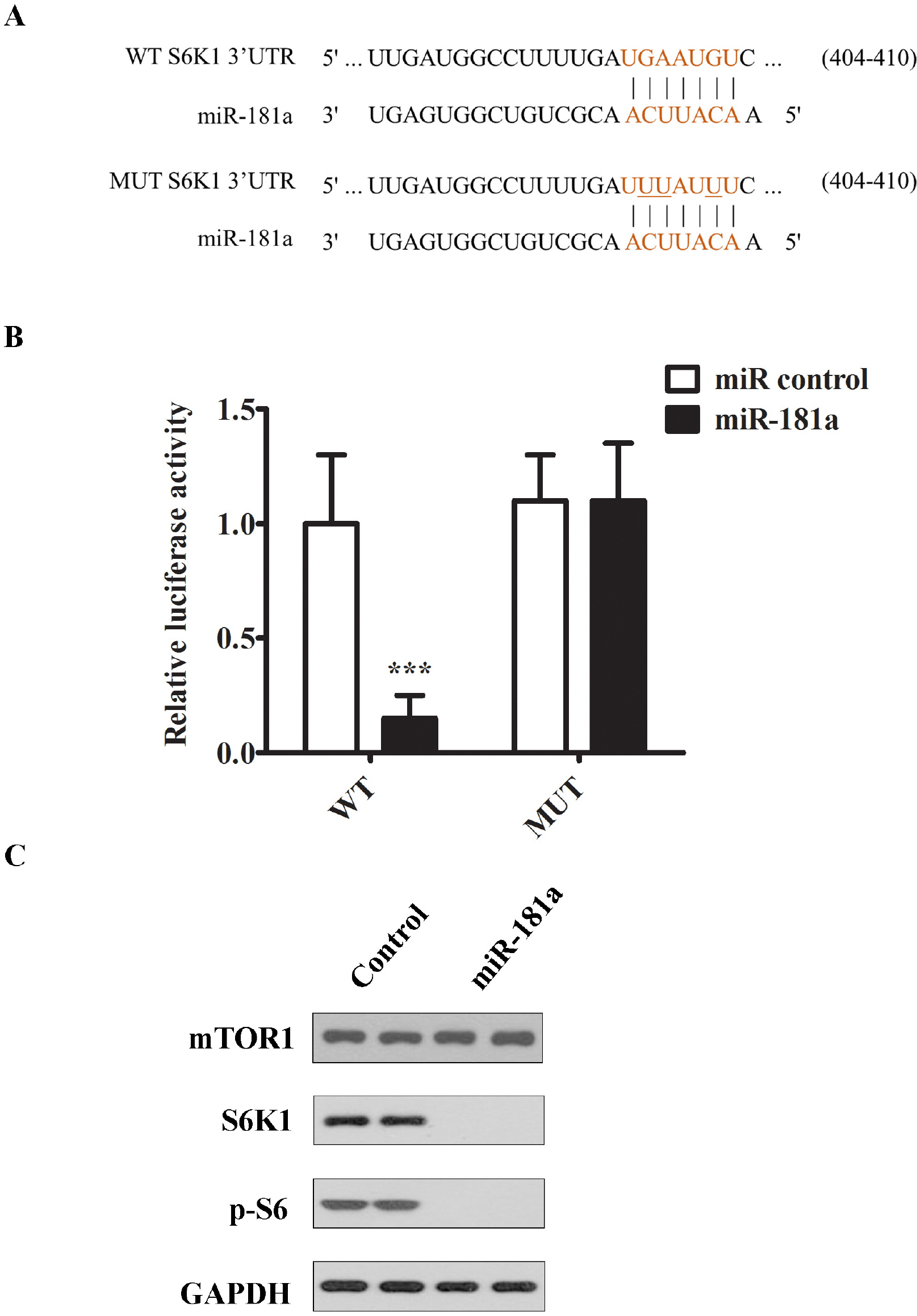
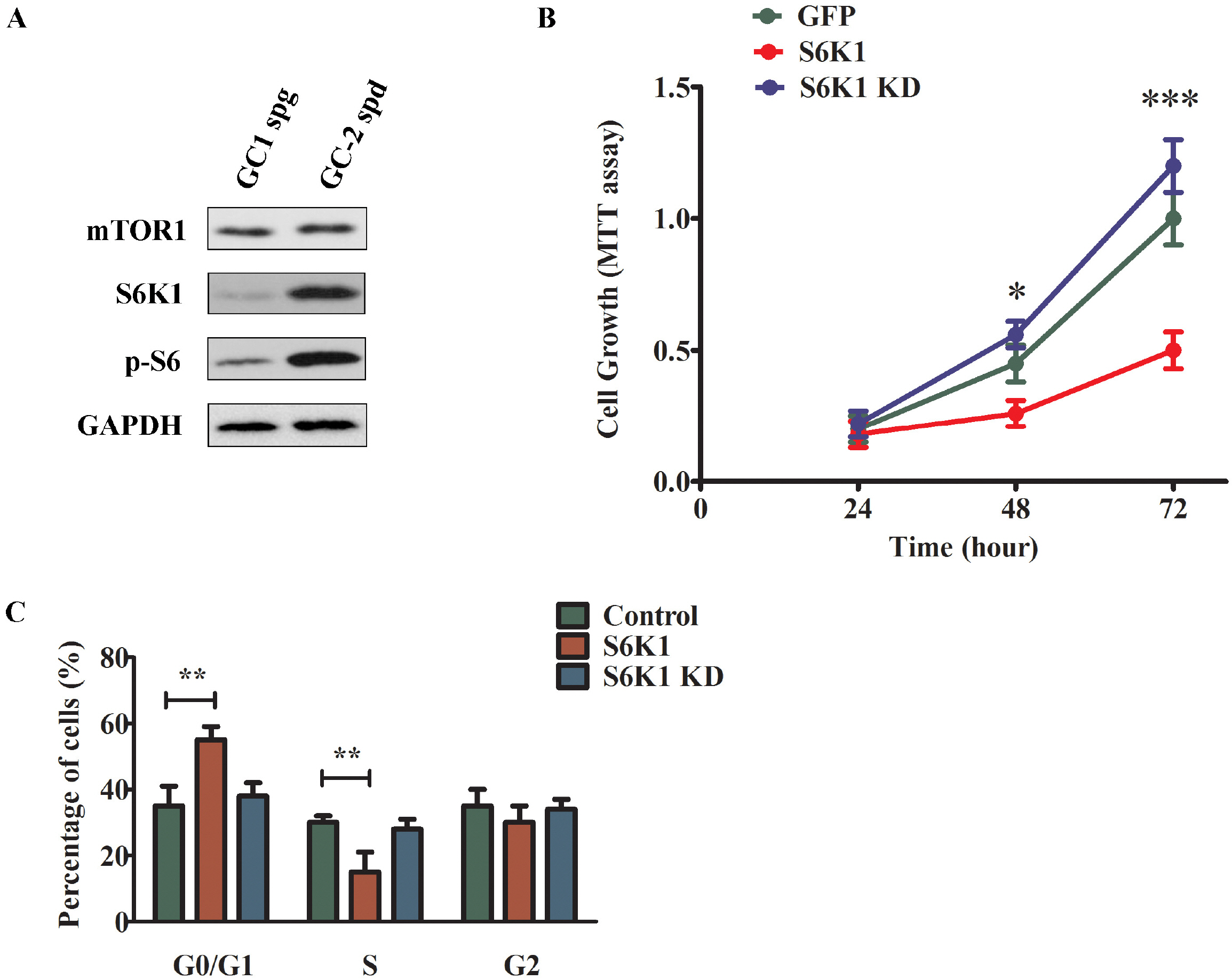
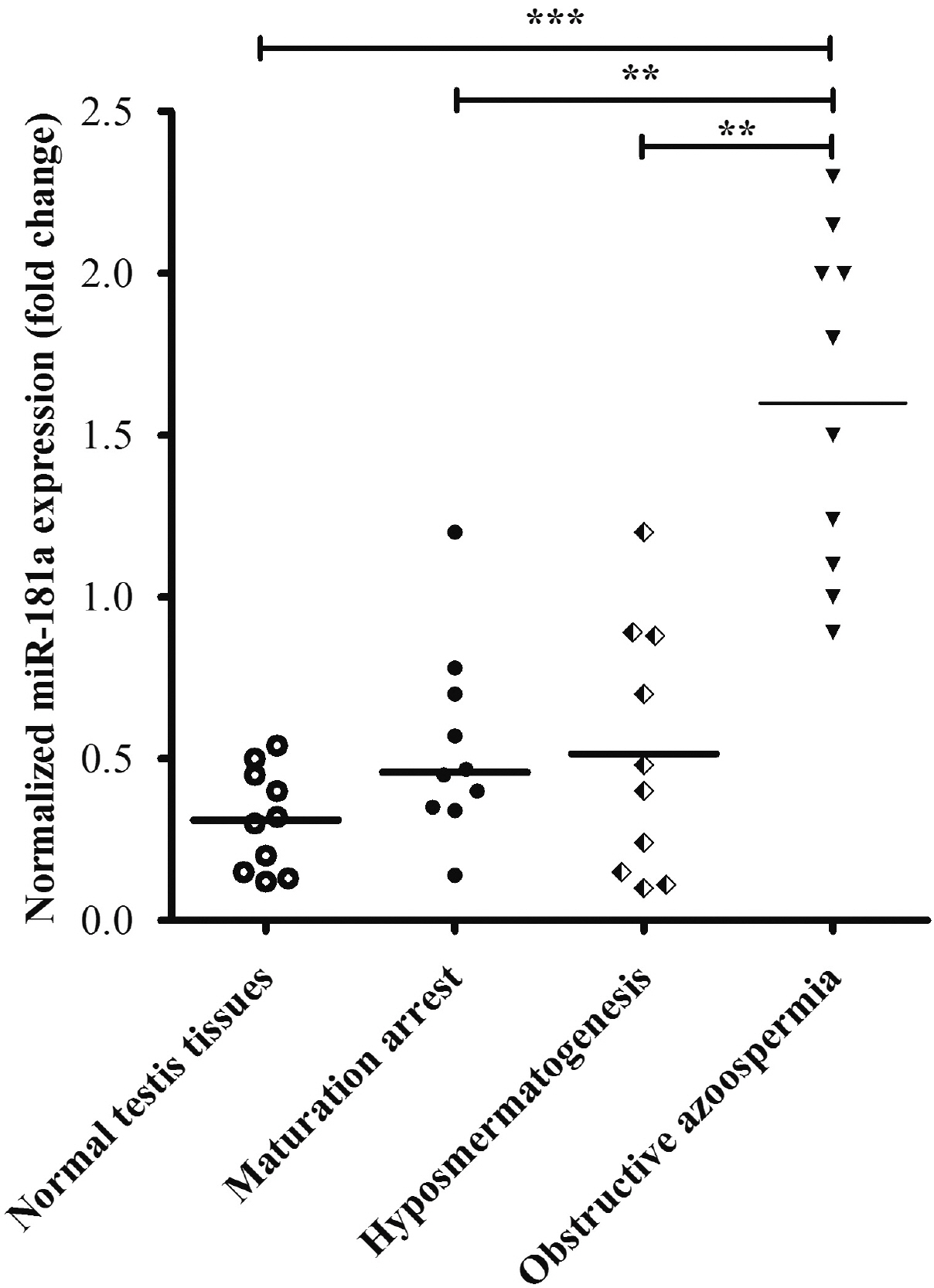
- Approximately 15% of couples suffer from infertility worldwide, and male factors contribute to about 30% of total sterility cases. However, there is little progress in treatments due to the obscured understanding of underlying mechanisms. Recently microRNAs have emerged as a key player in the process of spermatogenesis. Expression profiling of miR-181a was carried out in murine testes and spermatocyte culture system. In vitro cellular and biochemical assays were used to examine the effect of miR-181a and identify its target S6K1, as well as elucidate the function with chemical inhibitor of S6K1. Human testis samples analysis was employed to validate the findings. miR-181a level was upregulated during mouse spermatogenesis and knockdown of miR-181a attenuated the cell proliferation and G1/S arrest and increased the level of S6K1, which was identified as a downstream target of miR-181a. Overexpression of S6K1 also led to growth arrest of spermatocytes while inhibitor of S6K1 rescued the miR-181a knockdown-mediated cell proliferation defect. In human testis samples of azoospermia patients, low level of miR-181a was correlated with defects in the spermatogenic process. miR-181a is identified as a new regulator and high level of miR-181a contributes to spermatogenesis via targeting S6K1.
Keyword
Figure
Reference
-
References
1. Irvine DS. 1998; Epidemiology and aetiology of male infertility. Hum Reprod. 13 Suppl 1:33–44. DOI: 10.1093/humrep/13.suppl_1.33. PMID: 9663768.
Article2. Winters BR, Walsh TJ. 2014; The epidemiology of male infer-tility. Urol Clin North Am. 41:195–204. DOI: 10.1016/j.ucl.2013.08.006. PMID: 24286777.
Article3. Agarwal A, Mulgund A, Hamada A, Chyatte MR. 2015; A unique view on male infertility around the globe. Reprod Biol Endocrinol. 13:37. DOI: 10.1186/s12958-015-0032-1. PMID: 25928197. PMCID: PMC4424520.
Article4. Kumar N, Singh AK. 2015; Trends of male factor infertility, an important cause of infertility: a review of literature. J Hum Reprod Sci. 8:191–196. DOI: 10.4103/0974-1208.170370. PMID: 26752853. PMCID: PMC4691969.
Article5. Coutton C, Fissore RA, Palermo GD, Stouffs K, Touré A. 2016; Male infertility: genetics, mechanism, and therapies. Biomed Res Int. 2016:7372362. DOI: 10.1155/2016/7372362. PMID: 26942199. PMCID: PMC4753002.
Article6. Zheng W, Zou Z, Lin S, Chen X, Wang F, Li X, Dai J. 2019; Identification and functional analysis of spermatogenesis-associated gene modules in azoospermia by weighted gene coexpression network analysis. J Cell Biochem. 120:3934–3944. DOI: 10.1002/jcb.27677. PMID: 30269365.
Article7. Lee JY, Dada R, Sabanegh E, Carpi A, Agarwal A. 2011; Role of genetics in azoospermia. Urology. 77:598–601. DOI: 10.1016/j.urology.2010.10.001. PMID: 21195467.
Article8. Schlegel PN. 2004; Causes of azoospermia and their management. Reprod Fertil Dev. 16:561–572. DOI: 10.1071/RD03087. PMID: 15367371.
Article9. Wosnitzer MS, Goldstein M. 2014; Obstructive azoospermia. Urol Clin North Am. 41:83–95. DOI: 10.1016/j.ucl.2013.08.013. PMID: 24286769.
Article10. Baker K, Sabanegh E Jr. 2013; Obstructive azoospermia: recons-tructive techniques and results. Clinics (Sao Paulo). 68 Suppl 1:61–73. DOI: 10.6061/clinics/2013(Sup01)07. PMID: 23503955. PMCID: PMC3583161.
Article11. Jung JH, Seo JT. 2014; Empirical medical therapy in idiopathic male infertility: promise or panacea? Clin Exp Reprod Med. 41:108–114. DOI: 10.5653/cerm.2014.41.3.108. PMID: 25309854. PMCID: PMC4192450.
Article12. Gulino G, Stefanucci M, Antonucci M, Racioppi M, Sacco E, Pinto F, Bassi PF. 2014; Male infertility: non-surgical therapy. Urologia. 81:148–153. Italian. DOI: 10.5301/uro.5000087. PMID: 25198940.13. Firlej V, Barraud-Lange V, Fouchet P. 2012; Stem cell therapy for male infertility takes a step forward. Cell Stem Cell. 11:585–586. DOI: 10.1016/j.stem.2012.10.006. PMID: 23122284.
Article14. Khourdaji I, Lee H, Smith RP. 2018; Frontiers in hormone therapy for male infertility. Transl Androl Urol. 7(Suppl 3):S353–S366. DOI: 10.21037/tau.2018.04.03. PMID: 30159242. PMCID: PMC6087845.
Article15. Neto FT, Bach PV, Najari BB, Li PS, Goldstein M. 2016; Sper-matogenesis in humans and its affecting factors. Semin Cell Dev Biol. 59:10–26. DOI: 10.1016/j.semcdb.2016.04.009. PMID: 27143445.
Article16. Larson EL, Kopania EEK, Good JM. 2018; Spermatogenesis and the evolution of mammalian sex chromosomes. Trends Genet. 34:722–732. DOI: 10.1016/j.tig.2018.06.003. PMID: 30077434. PMCID: PMC6161750.
Article17. Tanaka H. 2007; Regulation of gene expression in spermatogenesis. Tanpakushitsu Kakusan Koso. 52(16 Suppl):2116–2123. Japanese. PMID: 21089281.18. Almog T, Naor Z. 2008; Mitogen activated protein kinases (MAPKs) as regulators of spermatogenesis and spermatozoa functions. Mol Cell Endocrinol. 282:39–44. DOI: 10.1016/j.mce.2007.11.011. PMID: 18177996.
Article19. Guillermet-Guibert J, Smith LB, Halet G, Whitehead MA, Pearce W, Rebourcet D, León K, Crépieux P, Nock G, Strömstedt M, Enerback M, Chelala C, Graupera M, Carroll J, Cosulich S, Saunders PT, Huhtaniemi I, Vanhae-sebroeck B. 2015; Novel role for p110β PI 3-kinase in male fertility through regulation of androgen receptor activity in Sertoli cells. PLoS Genet. 11:e1005304. DOI: 10.1371/journal.pgen.1005304. PMID: 26132308. PMCID: PMC4488938.
Article20. Dias VL, Rajpert-De Meyts E, McLachlan R, Loveland KL. 2009; Analysis of activin/TGFB-signaling modulators within the normal and dysfunctional adult human testis reveals evidence of altered signaling capacity in a subset of semino-mas. Reproduction. 138:801–811. DOI: 10.1530/REP-09-0206. PMID: 19661148.
Article21. Xu H, Shen L, Chen X, Ding Y, He J, Zhu J, Wang Y, Liu X. 2016; mTOR/P70S6K promotes spermatogonia prolifera-tion and spermatogenesis in Sprague Dawley rats. Reprod Biomed Online. 32:207–217. DOI: 10.1016/j.rbmo.2015.11.007. PMID: 26706460.
Article22. Kotaja N. 2014; MicroRNAs and spermatogenesis. Fertil Steril. 101:1552–1562. DOI: 10.1016/j.fertnstert.2014.04.025. PMID: 24882619.
Article23. Chen X, Li X, Guo J, Zhang P, Zeng W. 2017; The roles of microRNAs in regulation of mammalian spermatogenesis. J Anim Sci Biotechnol. 8:35. DOI: 10.1186/s40104-017-0166-4. PMID: 28469844. PMCID: PMC5410700.
Article24. Gebert LFR, MacRae IJ. 2019; Regulation of microRNA function in animals. Nat Rev Mol Cell Biol. 20:21–37. DOI: 10.1038/s41580-018-0045-7. PMID: 30108335. PMCID: PMC6546304.25. Niu Z, Goodyear SM, Rao S, Wu X, Tobias JW, Avarbock MR, Brinster RL. 2011; MicroRNA-21 regulates the self-renewal of mouse spermatogonial stem cells. Proc Natl Acad Sci U S A. 108:12740–12745. DOI: 10.1073/pnas.1109987108. PMID: 21768389. PMCID: PMC3150879.
Article26. Smorag L, Zheng Y, Nolte J, Zechner U, Engel W, Pantakani DV. 2012; MicroRNA signature in various cell types of mouse spermatogenesis: evidence for stage-specifically expressed miRNA-221, -203 and -34b-5p mediated spermatogenesis regulation. Biol Cell. 104:677–692. DOI: 10.1111/boc.201200014. PMID: 22909339.
Article27. Huszar JM, Payne CJ. 2013; MicroRNA 146 (Mir146) modulates spermatogonial differentiation by retinoic acid in mice. Biol Reprod. 88:15. DOI: 10.1095/biolreprod.112.103747. PMID: 23221399. PMCID: PMC4434937.28. Tong MH, Mitchell DA, McGowan SD, Evanoff R, Griswold MD. 2012; Two miRNA clusters, Mir-17-92 (Mirc1) and Mir-106b-25 (Mirc3), are involved in the regulation of spermatogonial differentiation in mice. Biol Reprod. 86:72. DOI: 10.1095/biolreprod.111.096313. PMID: 22116806. PMCID: PMC3316268.29. Abu-Halima M, Hammadeh M, Schmitt J, Leidinger P, Keller A, Meese E, Backes C. 2013; Altered microRNA expression profiles of human spermatozoa in patients with different spermatogenic impairments. Fertil Steril. 99:1249–1255.e16. DOI: 10.1016/j.fertnstert.2012.11.054. PMID: 23312218.30. Huang CC, Yao HH. 2010; Inactivation of Dicer1 in Steroido-genic factor 1-positive cells reveals tissue-specific requirement for Dicer1 in adrenal, testis, and ovary. BMC Dev Biol. 10:66. DOI: 10.1186/1471-213X-10-66. PMID: 20540774. PMCID: PMC2897782.
Article31. Teng Y, Wang Y, Fu J, Cheng X, Miao S, Wang L. 2011; Cyclin T2: a novel miR-15a target gene involved in early spermato-genesis. FEBS Lett. 585:2493–2500. DOI: 10.1016/j.febslet.2011.06.031. PMID: 21740905.
Article32. Björk JK, Sandqvist A, Elsing AN, Kotaja N, Sistonen L. 2010; miR-18, a member of Oncomir-1, targets heat shock transcription factor 2 in spermatogenesis. Development. 137:3177–3184. DOI: 10.1242/dev.050955. PMID: 20724452.
Article33. McIver SC, Roman SD, Nixon B, McLaughlin EA. 2012; miRNA and mammalian male germ cells. Hum Reprod Update. 18:44–59. DOI: 10.1093/humupd/dmr041. PMID: 21989172.
Article34. Yan N, Lu Y, Sun H, Tao D, Zhang S, Liu W, Ma Y. 2007; A microarray for microRNA profiling in mouse testis tissues. Reproduction. 134:73–79. DOI: 10.1530/REP-07-0056. PMID: 17641090.
Article35. Shima H, Pende M, Chen Y, Fumagalli S, Thomas G, Kozma SC. 1998; Disruption of the p70(s6k)/p85(s6k) gene reveals a small mouse phenotype and a new functional S6 kinase. EMBO J. 17:6649–6659. DOI: 10.1093/emboj/17.22.6649. PMID: 9822608. PMCID: PMC1171010.
Article36. Pende M, Kozma SC, Jaquet M, Oorschot V, Burcelin R, Le Marchand-Brustel Y, Klumperman J, Thorens B, Thomas G. 2000; Hypoinsulinaemia, glucose intolerance and diminished beta-cell size in S6K1-deficient mice. Nature. 408:994–997. DOI: 10.1038/35050135. PMID: 11140689.
Article37. Ruvinsky I, Sharon N, Lerer T, Cohen H, Stolovich-Rain M, Nir T, Dor Y, Zisman P, Meyuhas O. 2005; Ribosomal protein S6 phosphorylation is a determinant of cell size and glucose homeostasis. Genes Dev. 19:2199–2211. DOI: 10.1101/gad.351605. PMID: 16166381. PMCID: PMC1221890.
Article38. Tanwar PS, Kaneko-Tarui T, Zhang L, Teixeira JM. 2012; Altered LKB1/AMPK/TSC1/TSC2/mTOR signaling causes disruption of Sertoli cell polarity and spermatogenesis. Hum Mol Genet. 21:4394–4405. DOI: 10.1093/hmg/dds272. PMID: 22791749. PMCID: PMC3459463.
Article39. Xie R, Lin X, Du T, Xu K, Shen H, Wei F, Hao W, Lin T, Lin X, Qin Y, Wang H, Chen L, Yang S, Yang J, Rong X, Yao K, Xiao D, Jia J, Sun Y. 2016; Targeted disruption of miR-17-92 impairs mouse spermatogenesis by activating mTOR signaling pathway. Medicine (Baltimore). 95:e2713. DOI: 10.1097/MD.0000000000002713. PMID: 26886608. PMCID: PMC4998608.
Article40. Boyer A, Girard M, Thimmanahalli DS, Levasseur A, Céleste C, Paquet M, Duggavathi R, Boerboom D. 2016; mTOR regulates gap junction alpha-1 protein trafficking in Sertoli cells and is required for the maintenance of spermatogenesis in mice. Biol Reprod. 95:13. DOI: 10.1095/biolreprod.115.138016. PMID: 27281705. PMCID: PMC5029431.
Article41. Henao-Mejia J, Williams A, Goff LA, Staron M, Licona-Limón P, Kaech SM, Nakayama M, Rinn JL, Flavell RA. 2013; The microRNA miR-181 is a critical cellular metabolic rheostat essential for NKT cell ontogenesis and lymphocyte development and homeostasis. Immunity. 38:984–997. DOI: 10.1016/j.immuni.2013.02.021. PMID: 23623381. PMCID: PMC3738211.
Article42. Meric-Bernstam F, Gonzalez-Angulo AM. 2009; Targeting the mTOR signaling network for cancer therapy. J Clin Oncol. 27:2278–2287. DOI: 10.1200/JCO.2008.20.0766. PMID: 19332717. PMCID: PMC2738634.
Article43. Park JA, Jin HO, Lee HN, Kim JH, Park IC, Noh WC, Chang YH, Hong YJ, Kim KC, Lee JK. 2015; S6K1 inhibition enhances the apoptotic cell death of breast cancer cells in response to Bcl-2/Bcl-xL inhibition by the downregulation of survivin. Oncol Lett. 10:829–834. DOI: 10.3892/ol.2015.3369. PMID: 26622578. PMCID: PMC4509134.
Article44. Choi HN, Jin HO, Kim JH, Hong SE, Kim HA, Kim EK, Lee JK, Park IC, Noh WC. 2013; Inhibition of S6K1 enhances glucose deprivation-induced cell death via downregulation of anti-apoptotic proteins in MCF-7 breast cancer cells. Biochem Biophys Res Commun. 432:123–128. DOI: 10.1016/j.bbrc.2013.01.074. PMID: 23376066.
Article45. Doscas ME, Williamson AJ, Usha L, Bogachkov Y, Rao GS, Xiao F, Wang Y, Ruby C, Kaufman H, Zhou J, Williams JW, Li Y, Xu X. 2014; Inhibition of p70 S6 kinase (S6K1) activity by A77 1726 and its effect on cell proliferation and cell cycle progress. Neoplasia. 16:824–834. DOI: 10.1016/j.neo.2014.08.006. PMID: 25379019. PMCID: PMC4212247.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- MicroRNA-181a-5p Curbs Osteogenic Differentiation and Bone Formation Partially Through Impairing Runx1-Dependent Inhibition of AIF-1 Transcription
- miR-205 and miR-200c: Predictive Micro RNAs for Lymph Node Metastasis in Triple Negative Breast Cancer
- Exosomes Derived from Human Adipose Mesenchymal Stem Cells Inhibits Fibrosis and Treats Oral Submucous Fibrosis via the miR-181a-5p/Smad2 Axis
- LINC00703 Acts as a Tumor Suppressor via Regulating miR-181a/KLF6 Axis in Gastric Cancer
- MicroRNA-21 promotes epithelial-mesenchymal transition and migration of human bronchial epithelial cells by targeting poly (ADP-ribose) polymerase-1 and activating PI3K/AKT signaling