Long-term outcomes of infliximab in a real-world multicenter cohort of patients with acute severe ulcerative colitis
- Affiliations
-
- 1Center for Crohn’s and Colitis, Department of Gastroenterology, Kyung Hee University Hospital, Kyung Hee University College of Medicine, Seoul, Korea
- 2Department of Internal Medicine and Liver Research Institute, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- 3Department of Gastroenterology, Hanyang University Guri Hospital, Hanyang University College of Medicine, Guri, Korea
- 4Department of Internal Medicine, St. Vincent’s Hospital, College of Medicine, The Catholic University of Korea, Suwon, Korea
- 5Department of Internal Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2518688
- DOI: http://doi.org/10.5217/ir.2020.00039
Abstract
- Background/Aims
Infliximab (IFX) has proven effective as rescue therapy in steroid-refractory acute severe ulcerative colitis (ASUC), however, the long-term real-world data are scarce. Our study aimed to assess the long-term treatment outcomes of IFX in a real-life cohort.
Methods
We established a multicenter retrospective cohort of hospitalized patients with ASUC, who met Truelove and Witt’s criteria and received intravenous corticosteroid (IVCS) or IFX during index hospitalization between 2006 and 2016 in 5 university hospitals in Korea. The cohort was systematically followed up until colectomy, death or last follow-up visit.
Results
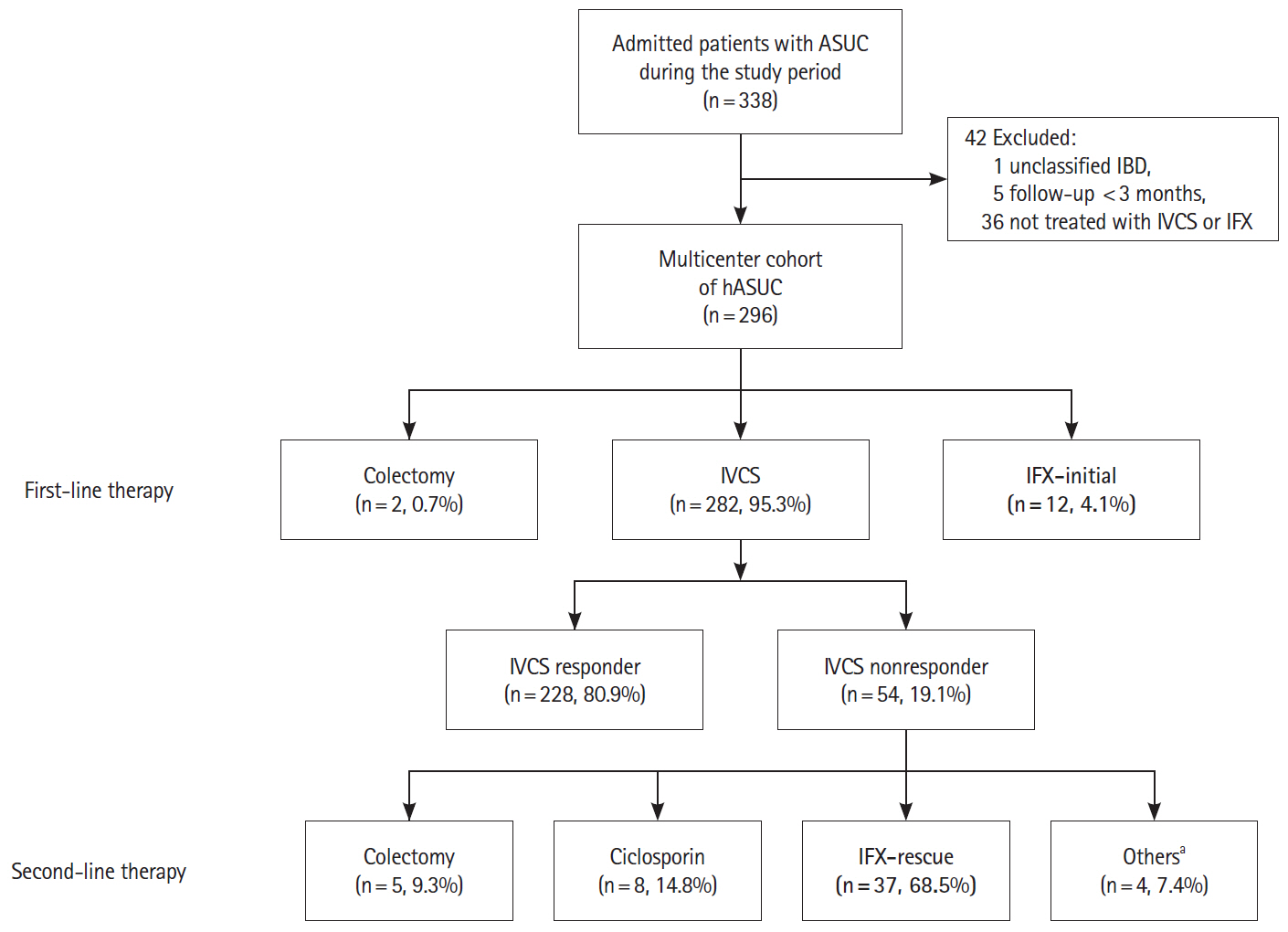
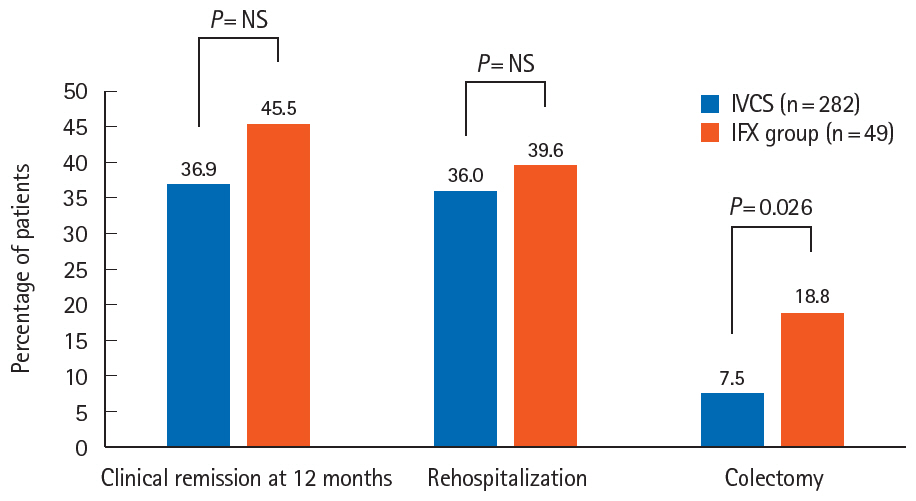
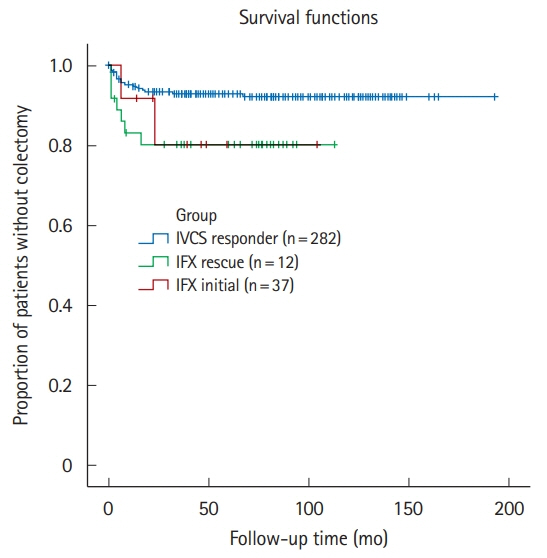
A total of 296 patients were followed up for a mean of 68.9 ± 44.0 months. During index hospitalization, 49 patients were treated with IFX; as rescue therapy for IVCS failure in 37 and as first-line medical therapy for ASUC in 12. All patients treated with IFX avoided colectomy during index hospitalization. The cumulative rates of rehospitalization and colectomy were 20.4% and 6.1% at 3 months and 39.6% and 18.8% at the end of follow-up, respectively. Patients treated with IFX presented with significantly shorter colectomy-free survival than IVCS responders (P= 0.04, log-rank test). Both cytomegalovirus colitis and Clostridioides difficile infection (CDI) were the significant predictors of colectomy in the overall study cohort (hazard ratios of 6.57 and 4.61, respectively). There were no fatalities.
Conclusions
Our real-world cohort study demonstrated that IFX is an effective therapeutic option in Korean patients with ASUC, irrespective of IFX indication. Aggressive vigilance for cytomegalovirus colitis and CDI is warranted for hospitalized patients with ASUC.
Keyword
Figure
Cited by 6 articles
-
Biomarker dynamics during infliximab salvage for acute severe ulcerative colitis: C-reactive protein (CRP)-lymphocyte ratio and CRP-albumin ratio are useful in predicting colectomy
Danny Con, Bridgette Andrew, Steven Nicolaides, Daniel R van Langenberg, Abhinav Vasudevan
Intest Res. 2022;20(1):101-113. doi: 10.5217/ir.2020.00146.Physician education can minimize inappropriate steroid use in patients with inflammatory bowel disease: the ACTION study
Yehyun Park, Chang Hwan Choi, Hyun Soo Kim, Hee Seok Moon, Do Hyun Kim, Jin Ju Kim, Dennis Teng, Dong Il Park
Intest Res. 2022;20(4):452-463. doi: 10.5217/ir.2021.00125.The role and prospect of tofacitinib in patients with ulcerative colitis
Jun Lee
Intest Res. 2023;21(1):168-169. doi: 10.5217/ir.2022.00098.Concomitant ankylosing spondylitis can increase the risk of biologics or small molecule therapies to control inflammatory bowel disease
Yu Kyung Jun, Hyuk Yoon, Seong-Joon Koh, A Hyeon Kim, Kwang Woo Kim, Jun Won Park, Hyun Jung Lee, Hyoun Woo Kang, Jong Pil Im, Young Soo Park, Joo Sung Kim
Intest Res. 2023;21(2):244-251. doi: 10.5217/ir.2022.00057.Reviewing not Homer’s
Iliad , but “Kai Bao Ben Cao ”: indigo dye—the past, present, and future
Yusuke Yoshimatsu, Tomohisa Sujino, Takanori Kanai
Intest Res. 2023;21(2):174-176. doi: 10.5217/ir.2022.00018.Risks of colorectal cancer and biliary cancer according to accompanied primary sclerosing cholangitis in Korean patients with ulcerative colitis: a nationwide population-based study
Eun Hye Oh, Ye-Jee Kim, Minju Kim, Seung Ha Park, Tae Oh Kim, Sang Hyoung Park
Intest Res. 2023;21(2):252-265. doi: 10.5217/ir.2022.00092.
Reference
-
1. Dinesen LC, Walsh AJ, Protic MN, et al. The pattern and outcome of acute severe colitis. J Crohns Colitis. 2010; 4:431–437.
Article2. Seah D, De Cruz P. Review article: the practical management of acute severe ulcerative colitis. Aliment Pharmacol Ther. 2016; 43:482–513.
Article3. Truelove SC, Witts LJ. Cortisone in ulcerative colitis: final report on a therapeutic trial. Br Med J. 1955; 2:1041–1048.4. Turner D, Walsh CM, Steinhart AH, Griffiths AM. Response to corticosteroids in severe ulcerative colitis: a systematic review of the literature and a meta-regression. Clin Gastroenterol Hepatol. 2007; 5:103–110.
Article5. Järnerot G, Hertervig E, Friis-Liby I, et al. Infliximab as rescue therapy in severe to moderately severe ulcerative colitis: a randomized, placebo-controlled study. Gastroenterology. 2005; 128:1805–1811.
Article6. Gustavsson A, Järnerot G, Hertervig E, et al. Clinical trial: colectomy after rescue therapy in ulcerative colitis: 3-year follow-up of the Swedish-Danish controlled infliximab study. Aliment Pharmacol Ther. 2010; 32:984–989.
Article7. Sjöberg M, Magnuson A, Björk J, et al. Infliximab as rescue therapy in hospitalised patients with steroid-refractory acute ulcerative colitis: a long-term follow-up of 211 Swedish patients. Aliment Pharmacol Ther. 2013; 38:377–387.
Article8. Laharie D, Bourreille A, Branche J, et al. Long-term outcome of patients with steroid-refractory acute severe UC treated with ciclosporin or infliximab. Gut. 2018; 67:237–243.
Article9. Park SH, Kim YM, Yang SK, et al. Clinical features and natural history of ulcerative colitis in Korea. Inflamm Bowel Dis. 2007; 13:278–283.
Article10. Fumery M, Singh S, Dulai PS, Gower-Rousseau C, Peyrin-Biroulet L, Sandborn WJ. Natural history of adult ulcerative colitis in population-based cohorts: a systematic review. Clin Gastroenterol Hepatol. 2018; 16:343–356.
Article11. Shi HY, Levy AN, Trivedi HD, Chan FKL, Ng SC, Ananthakrishnan AN. Ethnicity influences phenotype and outcomes in inflammatory bowel disease: a systematic review and meta-analysis of population-based studies. Clin Gastroenterol Hepatol. 2018; 16:190–197.
Article12. Ng WK, Wong SH, Ng SC. Changing epidemiological trends of inflammatory bowel disease in Asia. Intest Res. 2016; 14:111–119.
Article13. Seo H, Chang K, Lee SH, et al. Long-term outcomes of infliximab treatment and predictors of response in 195 patients with ulcerative colitis: a hospital-based cohort study from Korea. Scand J Gastroenterol. 2017; 52:857–863.
Article14. Minami N, Yoshino T, Matsuura M, et al. Tacrolimus or infliximab for severe ulcerative colitis: short-term and long-term data from a retrospective observational study. BMJ Open Gastroenterol. 2015; 2:e000021.
Article15. Lee KM, Jeen YT, Cho JY, et al. Efficacy, safety, and predictors of response to infliximab therapy for ulcerative colitis: a Korean multicenter retrospective study. J Gastroenterol Hepatol. 2013; 28:1829–1833.
Article16. Choi CH, Jung SA, Lee BI, et al. Diagnostic guideline of ulcerative colitis. Korean J Gastroenterol. 2009; 53:145–160.17. Magro F, Gionchetti P, Eliakim R, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis. 2017; 11:649–670.
Article18. Beswick L, Ye B, van Langenberg DR. Toward an algorithm for the diagnosis and management of CMV in patients with colitis. Inflamm Bowel Dis. 2016; 22:2966–2976.
Article19. McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018; 66:e1–e48.
Article20. Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987; 317:1625–1629.
Article21. Park SH, Kim YJ, Rhee KH, et al. A 30-year trend analysis in the epidemiology of inflammatory bowel disease in the Songpa-Kangdong District of Seoul, Korea in 1986-2015. J Crohns Colitis. 2019; 13:1410–1417.
Article22. Nalagatla N, Falloon K, Tran G, et al. Effect of accelerated infliximab induction on short- and long-term outcomes of acute severe ulcerative colitis: a retrospective multicenter study and meta-analysis. Clin Gastroenterol Hepatol. 2019; 17:502–509.
Article23. Lee HS, Yang SK, Soh JS, et al. Short- and long-term outcomes of acute severe ulcerative colitis in Korea: the 1999-2005 cohort. Inflamm Bowel Dis. 2015; 21:1825–1831.
Article24. Gibson DJ, Heetun ZS, Redmond CE, et al. An accelerated infliximab induction regimen reduces the need for early colectomy in patients with acute severe ulcerative colitis. Clin Gastroenterol Hepatol. 2015; 13:330–335.
Article25. Seah D, Choy MC, Gorelik A, et al. Examining maintenance care following infliximab salvage therapy for acute severe ulcerative colitis. J Gastroenterol Hepatol. 2018; 33:226–231.
Article26. Gisbert JP, Chaparro M. Predictors of primary response to biologic treatment (anti-TNF, vedolizumab and ustekinumab) in patients with inflammatory bowel disease: from basic science to clinical practice. J Crohns Colitis. 2020; 14:694–709.
Article27. Oh SJ, Lee CK, Kim YW, et al. True cytomegalovirus colitis is a poor prognostic indicator in patients with ulcerative colitis flares: the 10-year experience of an academic referral inflammatory bowel disease center. Scand J Gastroenterol. 2019; 54:976–983.
Article28. Law CC, Tariq R, Khanna S, Murthy S, McCurdy JD. Systematic review with meta-analysis: the impact of Clostridium difficile infection on the short- and long-term risks of colectomy in inflammatory bowel disease. Aliment Pharmacol Ther. 2017; 45:1011–1020.
Article29. Colombel JF, Rutgeerts P, Reinisch W, et al. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology. 2011; 141:1194–1201.
Article30. Saigusa K, Matsuoka K, Sugimoto S, et al. Ulcerative colitis endoscopic index of severity is associated with long-term prognosis in ulcerative colitis patients treated with infliximab. Dig Endosc. 2016; 28:665–670.
Article31. Nasuno M, Miyakawa M, Tanaka H, Motoya S. Short- and long-term outcomes of infliximab treatment for steroid-refractory ulcerative colitis and related prognostic factors: a single-center retrospective study. Digestion. 2017; 95:67–71.
Article32. Berinstein JA, Steiner CA, Regal RE, et al. Efficacy of induction therapy with high-intensity tofacitinib in 4 patients with acute severe ulcerative colitis. Clin Gastroenterol Hepatol. 2019; 17:988–990.
Article33. Kotwani P, Terdiman J, Lewin S. Tofacitinib for rescue therapy in acute severe ulcerative colitis: a real-world experience. J Crohns Colitis. 2020; 14:1026–1028.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- What to do when traditional rescue therapies fail in acute severe ulcerative colitis
- Long-term efficacy and safety of tofacitinib in patients with ulcerative colitis: 3-year results from a real-world study
- Comparison of Long-term Outcomes of Infliximab versus Adalimumab Treatment in Biologic-Naïve Patients with Ulcerative Colitis
- A Type II Segmental Vitiligo Developed under Infliximab Treatment for Ulcerative Colitis
- Golimumab Therapy in Ulcerative Colitis