J Stroke.
2021 May;23(2):223-233. 10.5853/jos.2020.04280.
Decreased Basal Ganglia Volume in Cerebral Amyloid Angiopathy
- Affiliations
-
- 1Department of Neurology, J.P. Kistler Stroke Research Center, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA
- 2Department of Neuroscience, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA
- 3Stroke Unit, Department of Neurology, University of Lille, INSERM U1171, CHU Lille, Lille, France
- 4Department of Radiology, Leiden University Medical Center, Leiden, the Netherlands
- KMID: 2516411
- DOI: http://doi.org/10.5853/jos.2020.04280
Abstract
- Background and Purpose
Cerebral amyloid angiopathy (CAA) is a common pathology of the leptomeningeal and cortical small vessels associated with hemorrhagic and non-hemorrhagic brain injury. Given previous evidence for CAA-related loss of cortical thickness and white matter volume, we hypothesized that CAA might also cause tissue loss in the basal ganglia.
Methods
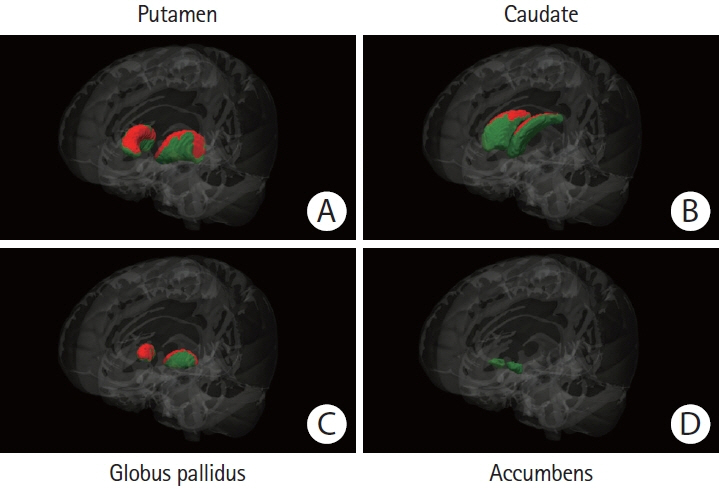
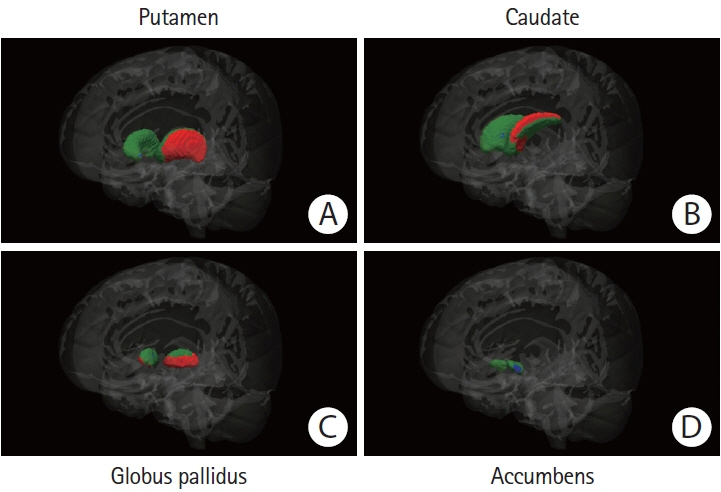
We compared basal ganglia volumes expressed as a percentage of total intracranial volume (pBGV) of non-demented patients with sporadic and hereditary CAA to age-matched healthy control (HC) and Alzheimer’s disease (AD) cohorts.
Results
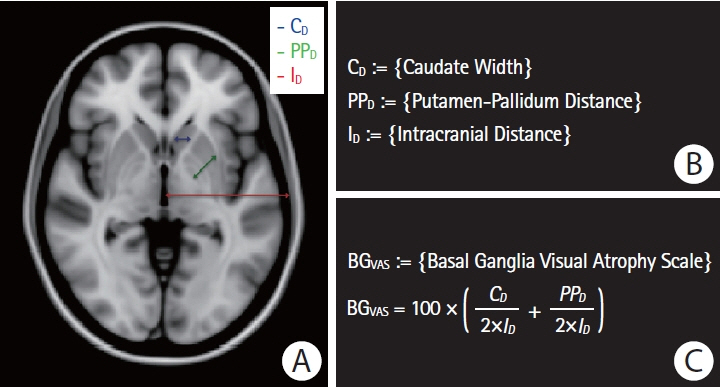
Patients with sporadic CAA had lower pBGV (n=80, 1.16%±0.14%) compared to HC (n=80, 1.30%±0.13%, P<0.0001) and AD patients (n=80, 1.23%±0.11%, P=0.001). Similarly, patients with hereditary CAA demonstrated lower pBGV (n=25, 1.26%±0.17%) compared to their matched HC (n=25, 1.36%±0.15%, P=0.036). Using a measurement of normalized basal ganglia width developed for analysis of clinical-grade magnetic resonance images, we found smaller basal ganglia width in patients with CAA-related lobar intracerebral hemorrhage (ICH; n=93, 12.35±1.47) compared to age-matched patients with hypertension-related deep ICH (n=93, 13.46±1.51, P<0.0001) or HC (n=93, 15.45±1.22, P<0.0001). Within the sporadic CAA research cohort, decreased basal ganglia volume was independently correlated with greater cortical gray matter atrophy (r=0.45, P<0.0001), increased basal ganglia fractional anisotropy (r=–0.36, P=0.001), and worse performance on language processing (r=0.35, P=0.003), but not with cognitive tests of executive function or processing speed.
Conclusions
These findings suggest an independent effect of CAA on basal ganglia tissue loss, indicating a novel mechanism for CAA-related brain injury and neurologic dysfunction.
Figure
Reference
-
References
1. Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013; 12:822–838.
Article2. Nitkunan A, Lanfranconi S, Charlton RA, Barrick TR, Markus HS. Brain atrophy and cerebral small vessel disease: a prospective follow-up study. Stroke. 2011; 42:133–138.3. Ikram MK, de Jong FJ, Vernooij MW, Hofman A, Niessen WJ, van der Lugt A, et al. Retinal vascular calibers associate differentially with cerebral gray matter and white matter atrophy. Alzheimer Dis Assoc Disord. 2013; 27:351–355.
Article4. Brundel M, van den Heuvel M, de Bresser J, Kappelle LJ, Biessels GJ; Utrecht Diabetic Encephalopathy Study Group. Cerebral cortical thickness in patients with type 2 diabetes. J Neurol Sci. 2010; 299:126–130.
Article5. Fotiadis P, van Rooden S, van der Grond J, Schultz A, Martinez- Ramirez S, Auriel E, et al. Cortical atrophy in patients with cerebral amyloid angiopathy: a case-control study. Lancet Neurol. 2016; 15:811–819.
Article6. Gurol ME, Viswanathan A, Gidicsin C, Hedden T, Martinez- Ramirez S, Dumas A, et al. Cerebral amyloid angiopathy burden associated with leukoaraiosis: a positron emission tomography/magnetic resonance imaging study. Ann Neurol. 2013; 73:529–536.
Article7. Fotiadis P, Reijmer YD, Van Veluw SJ, Martinez-Ramirez S, Karahanoglu FI, Gokcal E, et al. White matter atrophy in cerebral amyloid angiopathy. Neurology. 2020; 95:e554–e562.
Article8. Salat DH, Smith EE, Tuch DS, Benner T, Pappu V, Schwab KM, et al. White matter alterations in cerebral amyloid angiopathy measured by diffusion tensor imaging. Stroke. 2006; 37:1759–1764.
Article9. Reijmer YD, Fotiadis P, Martinez-Ramirez S, Salat DH, Schultz A, Shoamanesh A, et al. Structural network alterations and neurological dysfunction in cerebral amyloid angiopathy. Brain. 2015; 138(Pt 1):179–188.
Article10. Soontornniyomkij V, Lynch MD, Mermash S, Pomakian J, Badkoobehi H, Clare R, et al. Cerebral microinfarcts associated with severe cerebral beta-amyloid angiopathy. Brain Pathol. 2010; 20:459–467.11. Auriel E, Edlow BL, Reijmer YD, Fotiadis P, Ramirez-Martinez S, Ni J, et al. Microinfarct disruption of white matter structure: a longitudinal diffusion tensor analysis. Neurology. 2014; 83:182–188.
Article12. Dumas A, Dierksen GA, Gurol ME, Halpin A, Martinez- Ramirez S, Schwab K, et al. Functional magnetic resonance imaging detection of vascular reactivity in cerebral amyloid angiopathy. Ann Neurol. 2012; 72:76–81.
Article13. Peca S, McCreary CR, Donaldson E, Kumarpillai G, Shobha N, Sanchez K, et al. Neurovascular decoupling is associated with severity of cerebral amyloid angiopathy. Neurology. 2013; 81:1659–1665.
Article14. Mink JW. The basal ganglia and involuntary movements: impaired inhibition of competing motor patterns. Arch Neurol. 2003; 60:1365–1368.15. Foerde K, Shohamy D. The role of the basal ganglia in learning and memory: insight from Parkinson’s disease. Neurobiol Learn Mem. 2011; 96:624–636.
Article16. Booth JR, Wood L, Lu D, Houk JC, Bitan T. The role of the basal ganglia and cerebellum in language processing. Brain Res. 2007; 1133:136–144.
Article17. Tanaka SC, Doya K, Okada G, Ueda K, Okamoto Y, Yamawaki S. Prediction of immediate and future rewards differentially recruits cortico-basal ganglia loops. Nat Neurosci. 2004; 7:887–893.
Article18. Ashar YK, Andrews-Hanna JR, Dimidjian S, Wager TD. Empathic care and distress: predictive brain markers and dissociable brain systems. Neuron. 2017; 94:1263–1273.
Article19. Tinaz S, Courtney MG, Stern CE. Focal cortical and subcortical atrophy in early Parkinson’s disease. Mov Disord. 2011; 26:436–441.
Article20. Cordato NJ, Halliday GM, Harding AJ, Hely MA, Morris JG. Regional brain atrophy in progressive supranuclear palsy and Lewy body disease. Ann Neurol. 2000; 47:718–728.
Article21. Aylward EH, Li Q, Stine OC, Ranen N, Sherr M, Barta PE, et al. Longitudinal change in basal ganglia volume in patients with Huntington’s disease. Neurology. 1997; 48:394–399.
Article22. Hanganu A, Provost JS, Monchi O. Neuroimaging studies of striatum in cognition part II: Parkinson’s disease. Front Syst Neurosci. 2015; 9:138.
Article23. Greenberg SM, Charidimou A. Diagnosis of cerebral amyloid angiopathy: evolution of the Boston criteria. Stroke. 2018; 49:491–497.24. Jack CR Jr, Bernstein MA, Fox NC, Thompson P, Alexander G, Harvey D, et al. The Alzheimer’s Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging. 2008; 27:685–691.
Article25. van Rooden S, van der Grond J, van den Boom R, Haan J, Linn J, Greenberg SM, et al. Descriptive analysis of the Boston criteria applied to a Dutch-type cerebral amyloid angiopathy population. Stroke. 2009; 40:3022–3027.
Article26. Pasi M, Boulouis G, Fotiadis P, Auriel E, Charidimou A, Haley K, et al. Distribution of lacunes in cerebral amyloid angiopathy and hypertensive small vessel disease. Neurology. 2017; 88:2162–2168.
Article27. Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000; 97:11050–11055.
Article28. Lee MJ, Kim TH, Kim SJ, Kim BK, Mun CW, Lee JH. Quantitative validation of a visual rating scale for defining high-iron putamen in patients with multiple system atrophy. Front Neurol. 2019; 10:1014.
Article29. Case NF, Charlton A, Zwiers A, Batool S, McCreary CR, Hogan DB, et al. Cerebral amyloid angiopathy is associated with executive dysfunction and mild cognitive impairment. Stroke. 2016; 47:2010–2016.
Article30. Selden N, Mesulam MM, Geula C. Human striatum: the distribution of neurofibrillary tangles in Alzheimer’s disease. Brain Res. 1994; 648:327–331.
Article31. Gray F, Dubas F, Roullet E, Escourolle R. Leukoencephalopathy in diffuse hemorrhagic cerebral amyloid angiopathy. Ann Neurol. 1985; 18:54–59.
Article32. Smith EE, Nandigam KR, Chen YW, Jeng J, Salat D, Halpin A, et al. MRI markers of small vessel disease in lobar and deep hemispheric intracerebral hemorrhage. Stroke. 2010; 41:1933–1938.
Article33. Fisher CM. Pathological observations in hypertensive cerebral hemorrhage. J Neuropathol Exp Neurol. 1971; 30:536–550.
Article34. Werring DJ, Clark CA, Barker GJ, Thompson AJ, Miller DH. Diffusion tensor imaging of lesions and normal-appearing white matter in multiple sclerosis. Neurology. 1999; 52:1626–1632.
Article35. Douaud G, Behrens TE, Poupon C, Cointepas Y, Jbabdi S, Gaura V, et al. In vivo evidence for the selective subcortical degeneration in Huntington’s disease. Neuroimage. 2009; 46:958–966.
Article36. Hasan KM, Halphen C, Kamali A, Nelson FM, Wolinsky JS, Narayana PA. Caudate nuclei volume, diffusion tensor metrics, and T(2) relaxation in healthy adults and relapsing-remitting multiple sclerosis patients: implications for understanding gray matter degeneration. J Magn Reson Imaging. 2009; 29:70–77.37. Aylward EH, Harrington DL, Mills JA, Nopoulos PC, Ross CA, Long JD, et al. Regional atrophy associated with cognitive and motor function in prodromal Huntington disease. J Huntingtons Dis. 2013; 2:477–489.
Article38. Gurol ME, Irizarry MC, Smith EE, Raju S, Diaz-Arrastia R, Bottiglieri T, et al. Plasma beta-amyloid and white matter lesions in AD, MCI, and cerebral amyloid angiopathy. Neurology. 2006; 66:23–29.39. Maat-Schieman ML, Radder CM, van Duinen SG, Haan J, Roos RA. Hereditary cerebral hemorrhage with amyloidosis (Dutch): a model for congophilic plaque formation without neurofibrillary pathology. Acta Neuropathol. 1994; 88:371–378.
Article40. Greenberg SM, Al-Shahi Salman R, Biessels GJ, van Buchem M, Cordonnier C, Lee JM, et al. Outcome markers for clinical trials in cerebral amyloid angiopathy. Lancet Neurol. 2014; 13:419–428.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Spontaneous Bilateral Basal Ganglia Hemorrhage: Case Report
- A Case of Cerebral Amyloid Angiopathy-related Intracerebral Hemorrhage
- Multiple Recurrent Cerebral Hemorrhages Related to Cerebral Amyloid Angiopathy with Arterial Hypertension
- Cerebral Amyloid Angiopathy: An Undeniable Small Vessel Disease
- Patterns of Cerebrospinal Fluid Biomarkers and Amyloid Positron Emission Tomography in a Patient with Cerebral Amyloid Angiopathy-Related Inflammation