J Stroke.
2024 Jan;26(1):1-12. 10.5853/jos.2023.01942.
Cerebral Amyloid Angiopathy: An Undeniable Small Vessel Disease
- Affiliations
-
- 1Department of Neurology and Institute of Neurology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- KMID: 2551343
- DOI: http://doi.org/10.5853/jos.2023.01942
Abstract
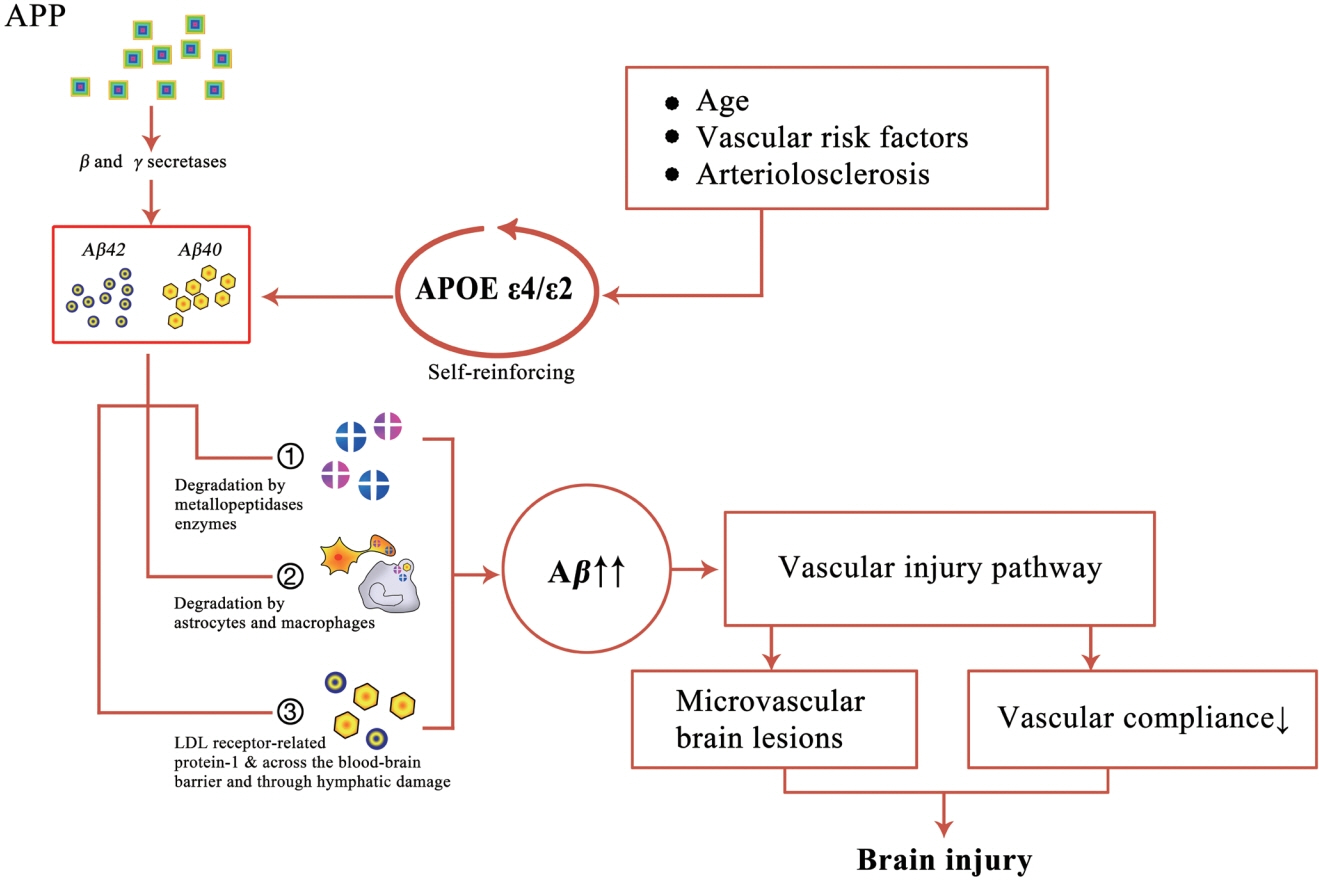
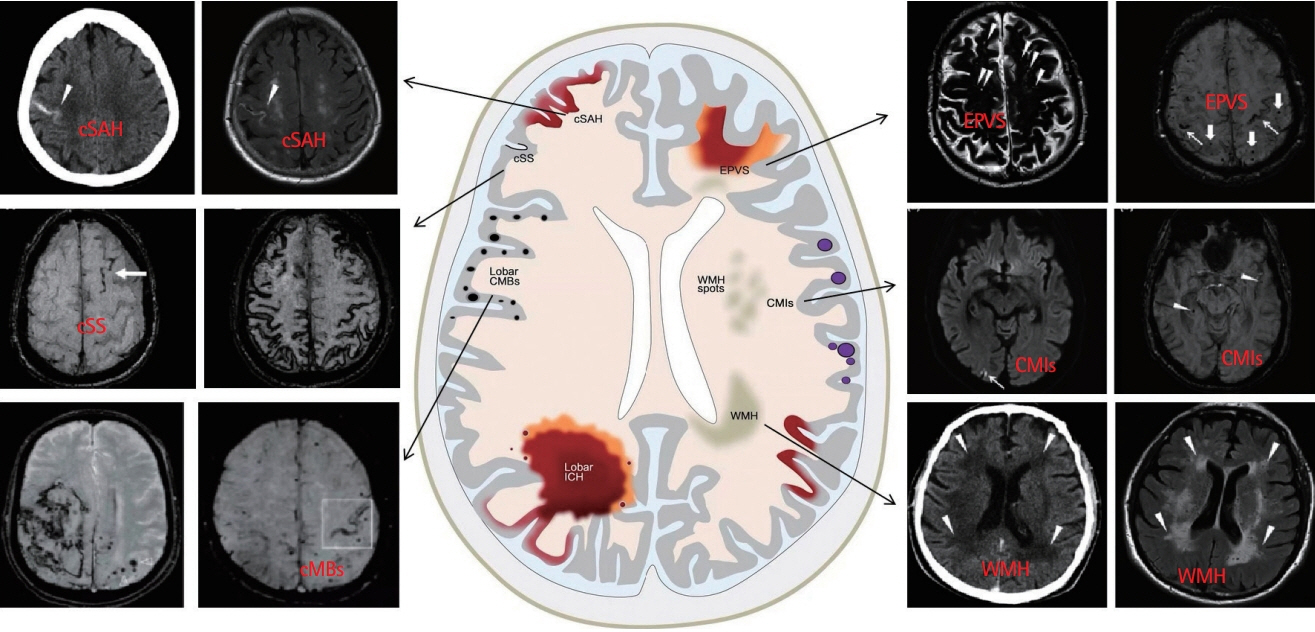
- Cerebral amyloid angiopathy (CAA) has been proven to be the most common pathological change in cerebral small vessel disease except arteriosclerosis. In recent years, with the discovery of imaging technology and new imaging markers, the diagnostic rate of CAA has greatly improved. CAA plays an important role in non-hypertensive cerebral hemorrhage and cognitive decline. This review comprehensively describes the etiology, epidemiology, pathophysiological mechanisms, clinical features, imaging manifestations, imaging markers, diagnostic criteria, and treatment of CAA to facilitate its diagnosis and treatment and reduce mortality.
Keyword
Figure
Reference
-
References
1. Zhang-Nunes SX, Maat-Schieman ML, van Duinen SG, Roos RA, Frosch MP, Greenberg SM. The cerebral beta-amyloid angiopathies: hereditary and sporadic. Brain Pathol. 2006; 16:30–39.2. Viswanathan A, Greenberg SM. Cerebral amyloid angiopathy in the elderly. Ann Neurol. 2011; 70:871–880.3. Boyle PA, Yu L, Nag S, Leurgans S, Wilson RS, Bennett DA, et al. Cerebral amyloid angiopathy and cognitive outcomes in community-based older persons. Neurology. 2015; 85:1930–1936.4. Banerjee G, Carare R, Cordonnier C, Greenberg SM, Schneider JA, Smith EE, et al. The increasing impact of cerebral amyloid angiopathy: essential new insights for clinical practice. J Neurol Neurosurg Psychiatry. 2017; 88:982–994.5. Jaunmuktane Z, Quaegebeur A, Taipa R, Viana-Baptista M, Barbosa R, Koriath C, et al. Evidence of amyloid-β cerebral amyloid angiopathy transmission through neurosurgery. Acta Neuropathol. 2018; 135:671–679.6. Banerjee G, Samra K, Adams ME, Jaunmuktane Z, Parry-Jones AR, Grieve J, et al. Iatrogenic cerebral amyloid angiopathy: an emerging clinical phenomenon. J Neurol Neurosurg Psychiatry. 2022; 93:693–700.7. Yamada M. Cerebral amyloid angiopathy: emerging concepts. J Stroke. 2015; 17:17–30.8. Kamp JA, Moursel LG, Haan J, Terwindt GM, Lesnik Oberstein SA, van Duinen SG, et al. Amyloid β in hereditary cerebral hemorrhage with amyloidosis-Dutch type. Rev Neurosci. 2014; 25:641–651.9. Ristori E, Donnini S, Ziche M. New insights into blood-brain barrier maintenance: the homeostatic role of β-amyloid precursor protein in cerebral vasculature. Front Physiol. 2020; 11:1056.10. Levy E, Lopez-Otin C, Ghiso J, Geltner D, Frangione B. Stroke in icelandic patients with hereditary amyloid angiopathy is related to a mutation in the cystatin C gene, an inhibitor of cysteine proteases. J Exp Med. 1989; 169:1771–1778.11. Ghiso J, Jensson O, Frangione B. Amyloid fibrils in hereditary cerebral hemorrhage with amyloidosis of Icelandic type is a variant of gamma-trace basic protein (cystatin C). Proc Natl Acad Sci U S A. 1986; 83:2974–2978.12. Koemans EA, Castello JP, Rasing I, Abramson JR, Voigt S, Perosa V, et al. Sex differences in onset and progression of cerebral amyloid angiopathy. Stroke. 2023; 54:306–314.13. Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010; 9:689–701.14. Thal DR, Walter J, Saido TC, Fändrich M. Neuropathology and biochemistry of Aβ and its aggregates in Alzheimer’s disease. Acta Neuropathol. 2015; 129:167–182.15. DeSimone CV, Graff-Radford J, El-Harasis MA, Rabinstein AA, Asirvatham SJ, Holmes DR Jr. Cerebral amyloid angiopathy: diagnosis, clinical implications, and management strategies in atrial fibrillation. J Am Coll Cardiol. 2017; 70:1173–1182.16. Thal DR, Ghebremedhin E, Rüb U, Yamaguchi H, Del Tredici K, Braak H. Two types of sporadic cerebral amyloid angiopathy. J Neuropathol Exp Neurol. 2002; 61:282–293.17. Thal DR, Ghebremedhin E, Orantes M, Wiestler OD. Vascular pathology in Alzheimer disease: correlation of cerebral amyloid angiopathy and arteriosclerosis/lipohyalinosis with cognitive decline. J Neuropathol Exp Neurol. 2003; 62:1287–1301.18. Charidimou A, Meegahage R, Fox Z, Peeters A, Vandermeeren Y, Laloux P, et al. Enlarged perivascular spaces as a marker of underlying arteriopathy in intracerebral haemorrhage: a multicentre MRI cohort study. J Neurol Neurosurg Psychiatry. 2013; 84:624–629.19. Falcone GJ, Woo D. Genetics of spontaneous intracerebral hemorrhage. Stroke. 2017; 48:3420–3424.20. Carpenter AM, Singh IP, Gandhi CD, Prestigiacomo CJ. Genetic risk factors for spontaneous intracerebral haemorrhage. Nat Rev Neurol. 2016; 12:40–49.21. Serrano-Pozo A, Das S, Hyman BT. APOE and Alzheimer’s disease: advances in genetics, pathophysiology, and therapeutic approaches. Lancet Neurol. 2021; 20:68–80.22. Rannikmäe K, Samarasekera N, Martînez-Gonzâlez NA, AlShahi Salman R, Sudlow CL. Genetics of cerebral amyloid angiopathy: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2013; 84:901–908.23. Lamar M, Yu L, Rubin LH, James BD, Barnes LL, Farfel JM, et al. APOE genotypes as a risk factor for age-dependent accumulation of cerebrovascular disease in older adults. Alzheimers Dement. 2019; 15:258–266.24. Chu S, Xu F, Su Y, Chen H, Cheng X. Cerebral amyloid angiopathy (CAA)-related inflammation: comparison of inflammatory CAA and amyloid-β-related angiitis. J Alzheimers Dis. 2016; 51:525–532.25. Chwalisz BK. Cerebral amyloid angiopathy and related inflammatory disorders. J Neurol Sci. 2021; 424:117425.26. Kirshner HS, Bradshaw M. The inflammatory form of cerebral amyloid angiopathy or “cerebral amyloid angiopathy-related inflammation” (CAARI). Curr Neurol Neurosci Rep. 2015; 15:54.27. Theodorou A, Palaiodimou L, Safouris A, Kargiotis O, Psychogios K, Kotsali-Peteinelli V, et al. Cerebral amyloid angiopathy-related inflammation: a single-center experience and a literature review. J Clin Med. 2022; 11:6731.28. Scolding NJ, Joseph F, Kirby PA, Mazanti I, Gray F, Mikol J, et al. Abeta-related angiitis: primary angiitis of the central nervous system associated with cerebral amyloid angiopathy. Brain. 2005; 128:500–515.29. Auriel E, Greenberg SM. The pathophysiology and clinical presentation of cerebral amyloid angiopathy. Curr Atheroscler Rep. 2012; 14:343–350.30. Renard D, Tatu L, Collombier L, Wacongne A, Ayrignac X, Charif M, et al. Cerebral amyloid angiopathy and cerebral amyloid angiopathy-related inflammation: comparison of hemorrhagic and DWI MRI features. J Alzheimers Dis. 2018; 64:1113–1121.31. Zhou H, Gao F, Yang X, Lin T, Li Z, Wang Q, et al. Endothelial BACE1 impairs cerebral small vessels via tight junctions and eNOS. Circ Res. 2022; 130:1321–1341.32. Ihara M. Endothelial BACE1: bridging the gap between hypertension and Alzheimer’s disease. Circ Res. 2022; 130:1342–1344.33. Koelsch G. BACE1 function and inhibition: implications of intervention in the amyloid pathway of Alzheimer’s disease pathology. Molecules. 2017; 22:1723.34. Charidimou A, Boulouis G, Gurol ME, Ayata C, Bacskai BJ, Frosch MP, et al. Emerging concepts in sporadic cerebral amyloid angiopathy. Brain. 2017; 140:1829–1850.35. Lovelock CE, Molyneux AJ, Rothwell PM; Oxford Vascular Study. Change in incidence and aetiology of intracerebral haemorrhage in Oxfordshire, UK, between 1981 and 2006: a population-based study. Lancet Neurol. 2007; 6:487–493.36. Chao CP, Kotsenas AL, Broderick DF. Cerebral amyloid angiopathy: CT and MR imaging findings. Radiographics. 2006; 26:1517–1531.37. Capron J. [Diagnosis and management of sporadic cerebral amyloid angiopathy]. Prat Neurol FMC. 2016; 7:239–249. French.38. Block F, Dafotakis M. Cerebral amyloid angiopathy in stroke medicine. Dtsch Arztebl Int. 2017; 114:37–42.39. Hirohata M, Yoshita M, Ishida C, Ikeda SI, Tamaoka A, Kuzuhara S, et al. Clinical features of non-hypertensive lobar intracerebral hemorrhage related to cerebral amyloid angiopathy. Eur J Neurol. 2010; 17:823–829.40. Charidimou A. Cerebral amyloid angiopathy-related transient focal neurological episodes (CAA-TFNEs): a well-defined clinical-radiological syndrome. J Neurol Sci. 2019; 406:116496.41. Charidimou A, Peeters A, Fox Z, Gregoire SM, Vandermeeren Y, Laloux P, et al. Spectrum of transient focal neurological episodes in cerebral amyloid angiopathy: multicentre magnetic resonance imaging cohort study and meta-analysis. Stroke. 2012; 43:2324–2330.42. Smith EE, Charidimou A, Ayata C, Werring DJ, Greenberg SM. Cerebral amyloid angiopathy-related transient focal neurologic episodes. Neurology. 2021; 97:231–238.43. Charidimou A, Linn J, Vernooij MW, Opherk C, Akoudad S, Baron JC, et al. Cortical superficial siderosis: detection and clinical significance in cerebral amyloid angiopathy and related conditions. Brain. 2015; 138:2126–2139.44. Charidimou A, Jäger RH, Fox Z, Peeters A, Vandermeeren Y, Laloux P, et al. Prevalence and mechanisms of cortical superficial siderosis in cerebral amyloid angiopathy. Neurology. 2013; 81:626–632.45. Vales-Montero M, García-Pastor A, Iglesias-Mohedano AM, Esteban-de Antonio E, Salgado-Cámara P, García-Domínguez JM, et al. Cerebral amyloid angiopathy-related transient focal neurological episodes: a transient ischemic attack mimic with an increased risk of intracranial hemorrhage. J Neurol Sci. 2019; 406:116452.46. Ellis RJ, Olichney JM, Thal LJ, Mirra SS, Morris JC, Beekly D, et al. Cerebral amyloid angiopathy in the brains of patients with Alzheimer’s disease: the CERAD experience, Part XV. Neurology. 1996; 46:1592–1596.47. Arvanitakis Z, Leurgans SE, Wang Z, Wilson RS, Bennett DA, Schneider JA. Cerebral amyloid angiopathy pathology and cognitive domains in older persons. Ann Neurol. 2011; 69:320–327.48. Durrani R, Wang M, Cox E, Irving E, Saad F, McCreary CR, et al. Mediators of cognitive impairment in cerebral amyloid angiopathy. Int J Stroke. 2023; 18:78–84.49. Rabin JS, Nichols E, La Joie R, Casaletto KB, Palta P, Dams-O’Connor K, et al. Cerebral amyloid angiopathy interacts with neuritic amyloid plaques to promote tau and cognitive decline. Brain. 2022; 145:2823–2833.50. Charidimou A, Martinez-Ramirez S, Reijmer YD, Oliveira-Filho J, Lauer A, Roongpiboonsopit D, et al. Total magnetic resonance imaging burden of small vessel disease in cerebral amyloid angiopathy: an imaging-pathologic study of concept validation. JAMA Neurol. 2016; 73:994–1001.51. Appleton JP, Woodhouse LJ, Adami A, Becker JL, Berge E, Cala LA, et al. Imaging markers of small vessel disease and brain frailty, and outcomes in acute stroke. Neurology. 2020; 94:e439–e452.52. Horn MJ, Gokcal E, Becker AJ, Das AS, Warren AD; Alzheimer Disease Neuroimaging Initiative; Schwab K, et al. Cerebellar atrophy and its implications on gait in cerebral amyloid angiopathy. J Neurol Neurosurg Psychiatry. 2022; 93:802–807.53. Pasi M, Boulouis G, Fotiadis P, Auriel E, Charidimou A, Haley K, et al. Distribution of lacunes in cerebral amyloid angiopathy and hypertensive small vessel disease. Neurology. 2017; 88:2162–2168.54. Werring DJ, Gregoire SM, Cipolotti L. Cerebral microbleeds and vascular cognitive impairment. J Neurol Sci. 2010; 299(1-2):P131–P135.55. Greenberg SM, Vernooij MW, Cordonnier C, Viswanathan A, Al-Shahi Salman R, Warach S, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009; 8:165–174.56. Polyakova TA, Levin OS. [Cerebral microbleeds in cerebrovascular and neurodegenerative diseases with cognitive impairment]. Zh Nevrol Psikhiatr Im S S Korsakova. 2016; 116:19–27. Russian.57. Vernooij MW, van der Lugt A, Ikram MA, Wielopolski PA, Niessen WJ, Hofman A, et al. Prevalence and risk factors of cerebral microbleeds: the Rotterdam Scan Study. Neurology. 2008; 70:1208–1214.58. Wang S, Zhang F, Huang P, Hong H, Jiaerken Y, Yu X, et al. Superficial white matter microstructure affects processing speed in cerebral small vessel disease. Hum Brain Mapp. 2022; 43:5310–5325.59. Chen J, Mikheev AV, Yu H, Gruen MD, Rusinek H, Ge Y; Alzheimer’s Disease Neuroimaging Initiative. Bilateral distance partition of periventricular and deep white matter hyperintensities: performance of the method in the aging brain. Acad Radiol. 2021; 28:1699–1708.60. Xu M, Cheng Y, Song Q, Yuan R, Zhang S, Hao Z, et al. Total burden of cerebral small vessel disease in recurrent ICH versus first-ever ICH. Aging Dis. 2019; 10:570–577.61. Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010; 341:c3666.62. Lampe L, Kharabian-Masouleh S, Kynast J, Arelin K, Steele CJ, Löffler M, et al. Lesion location matters: the relationships between white matter hyperintensities on cognition in the healthy elderly. J Cereb Blood Flow Metab. 2019; 39:36–43.63. Brown R, Low A, Markus HS. Rate of, and risk factors for, white matter hyperintensity growth: a systematic review and meta-analysis with implications for clinical trial design. J Neurol Neurosurg Psychiatry. 2021; 92:1271–1277.64. Charidimou A, Boulouis G, Fotiadis P, Xiong L, Ayres AM, Schwab KM, et al. Acute convexity subarachnoid haemorrhage and cortical superficial siderosis in probable cerebral amyloid angiopathy without lobar haemorrhage. J Neurol Neurosurg Psychiatry. 2018; 89:397–403.65. Raposo N, Charidimou A, Roongpiboonsopit D, Onyekaba M, Gurol ME, Rosand J, et al. Convexity subarachnoid hemorrhage in lobar intracerebral hemorrhage: a prognostic marker. Neurology. 2020; 94:e968–e977.66. Markus HS, de Leeuw FE. Cerebral small vessel disease: recent advances and future directions. Int J Stroke. 2023; 18:4–14.67. Yu L, Hu X, Li H, Zhao Y. Perivascular spaces, glymphatic system and MR. Front Neurol. 2022; 13:844938.68. Evans TE, Knol MJ, Schwingenschuh P, Wittfeld K, Hilal S, Ikram MA, et al. Determinants of perivascular spaces in the general population: a pooled cohort analysis of individual participant data. Neurology. 2023; 100:e107–e122.69. Ramirez J, Berezuk C, McNeely AA, Gao F, McLaurin J, Black SE. Imaging the perivascular space as a potential biomarker of neurovascular and neurodegenerative diseases. Cell Mol Neurobiol. 2016; 36:289–299.70. Inglese M, Bomsztyk E, Gonen O, Mannon LJ, Grossman RI, Rusinek H. Dilated perivascular spaces: hallmarks of mild traumatic brain injury. AJNR Am J Neuroradiol. 2005; 26:719–724.71. Kwee RM, Kwee TC. Virchow-Robin spaces at MR imaging. Radiographics. 2007; 27:1071–1086.72. Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013; 12:822–838.73. Passiak BS, Liu D, Kresge HA, Cambronero FE, Pechman KR, Osborn KE, et al. Perivascular spaces contribute to cognition beyond other small vessel disease markers. Neurology. 2019; 92:e1309–e1321.74. Perosa V, Oltmer J, Munting LP, Freeze WM, Auger CA, Scherlek AA, et al. Perivascular space dilation is associated with vascular amyloid-β accumulation in the overlying cortex. Acta Neuropathol. 2022; 143:331–348.75. Montagne A, Nation DA, Sagare AP, Barisano G, Sweeney MD, Chakhoyan A, et al. APOE4 leads to blood-brain barrier dysfunction predicting cognitive decline. Nature. 2020; 581:71–76.76. Smith EE, Schneider JA, Wardlaw JM, Greenberg SM. Cerebral microinfarcts: the invisible lesions. Lancet Neurol. 2012; 11:272–282.77. Corrada MM, Sonnen JA, Kim RC, Kawas CH. Microinfarcts are common and strongly related to dementia in the oldest-old: the 90+ study. Alzheimers Dement. 2016; 12:900–908.78. van Veluw SJ, Charidimou A, van der Kouwe AJ, et al. Microbleed and microinfarct detection in amyloid angiopathy: a high-resolution MRI-histopathology study. Brain. 2016; 139:3151–3162.79. van Dalen JW, Scuric EE, van Veluw SJ, Caan MW. Cortical microinfarcts detected in vivo on 3 Tesla MRI: clinical and radiological correlates. Stroke. 2015; 46:255–257.80. Zwartbol MH, Rissanen I, Ghaznawi R, de Bresser J, Kuijf HJ, Blom K, et al. Cortical cerebral microinfarcts on 7T MRI: risk factors, neuroimaging correlates and cognitive functioning - the Medea-7T study. J Cereb Blood Flow Metab. 2021; 41:3127–3138.81. Hilal S, Sikking E, Shaik MA, Chan QL, van Veluw SJ, Vrooman H, et al. Cortical cerebral microinfarcts on 3T MRI: a novel marker of cerebrovascular disease. Neurology. 2016; 87:1583–1590.82. Greenberg SM, Charidimou A. Diagnosis of cerebral amyloid angiopathy: evolution of the Boston criteria. Stroke. 2018; 49:491–497.83. Linn J, Halpin A, Demaerel P, Ruhland J, Giese AD, Dichgans M, et al. Prevalence of superficial siderosis in patients with cerebral amyloid angiopathy. Neurology. 2010; 74:1346–1350.84. Charidimou A, Boulouis G, Frosch MP, Baron JC, Pasi M, Albucher JF, et al. The Boston criteria version 2.0 for cerebral amyloid angiopathy: a multicentre, retrospective, MRI-neuropathology diagnostic accuracy study. Lancet Neurol. 2022; 21:714–725.85. Rodrigues MA, Samarasekera N, Lerpiniere C, Humphreys C, McCarron MO, White PM, et al. The Edinburgh CT and genetic diagnostic criteria for lobar intracerebral haemorrhage associated with cerebral amyloid angiopathy: model development and diagnostic test accuracy study. Lancet Neurol. 2018; 17:232–240.86. Chung KK, Anderson NE, Hutchinson D, Synek B, Barber PA. Cerebral amyloid angiopathy related inflammation: three case reports and a review. J Neurol Neurosurg Psychiatry. 2011; 82:20–26.87. Auriel E, Charidimou A, Gurol ME, Ni J, Van Etten ES, Martinez-Ramirez S, et al. Validation of clinicoradiological criteria for the diagnosis of cerebral amyloid angiopathy-related inflammation. JAMA Neurol. 2016; 73:197–202.88. Hemphill JC 3rd, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015; 46:2032–2060.89. Moullaali TJ, Wang X, Sandset EC, Woodhouse LJ, Law ZK, Arima H, et al. Early lowering of blood pressure after acute intracerebral haemorrhage: a systematic review and meta-analysis of individual patient data. J Neurol Neurosurg Psychiatry. 2022; 93:6–13.90. Rehni AK, Cho S, Quero HN, Shukla V, Zhang Z, Dong C, et al. Red Blood cell microparticles limit hematoma growth in intracerebral hemorrhage. Stroke. 2022; 53:3182–3191.91. Tsai HH, Hsieh YC, Lin JS, Kuo ZT, Ho CY, Chen CH, et al. Functional investigation of meningeal lymphatic system in experimental intracerebral hemorrhage. Stroke. 2022; 53:987–998.92. Xiong M, Jiang H, Serrano JR, Gonzales ER, Wang C, Gratuze M, et al. APOE immunotherapy reduces cerebral amyloid angiopathy and amyloid plaques while improving cerebrovascular function. Sci Transl Med. 2021; 13:eabd7522.93. Zhou G, Xiang T, Xu Y, He B, Wu L, Zhu G, et al. Fruquintinib/HMPL-013 ameliorates cognitive impairments and pathology in a mouse model of cerebral amyloid angiopathy (CAA). Eur J Pharmacol. 2023; 939:175446.94. Regenhardt RW, Thon JM, Das AS, Thon OR, Charidimou A, Viswanathan A, et al. Association between immunosuppressive treatment and outcomes of cerebral amyloid angiopathy-related inflammation. JAMA Neurol. 2020; 77:1261–1269.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Cerebral Amyloid Angiopathy-related Intracerebral Hemorrhage
- Multiple Recurrent Cerebral Hemorrhages Related to Cerebral Amyloid Angiopathy with Arterial Hypertension
- Concomitant Small Intracerebral Hemorrhage in a Patient with Cerebral Amyloid Angiopathy Mimicking Transient Ischemic Attack
- Lacunar Infarction and Small Vessel Disease: Pathology and Pathophysiology
- In vivo Image of Cerebral Amyloid Angiopathy in an Alzheimer's Disease Mouse Model