J Pathol Transl Med.
2021 May;55(3):202-211. 10.4132/jptm.2021.02.19.
Mismatch repair deficiency and clinicopathological characteristics in endometrial carcinoma: a systematic review and meta-analysis
- Affiliations
-
- 1Department of Pathology and Forensic Medicine, Faculty of Medicine, University of Kufa, Kufa, Iraq
- 2Al-Furat Al-Awsat Hospital, Kufa, Iraq
- 3School of Biomedical Sciences, Ulster University, Northern Ireland, UK
- KMID: 2515925
- DOI: http://doi.org/10.4132/jptm.2021.02.19
Abstract
- Background
Loss of mismatch repair (MMR) occurs frequently in endometrial carcinoma (EC) and is an important prognostic marker. However, the frequency of MMR deficiency (D-MMR) in EC remains inconclusive. This systematic review and meta-analysis addressed this inconsistency and evaluated related clinicopathology.
Methods
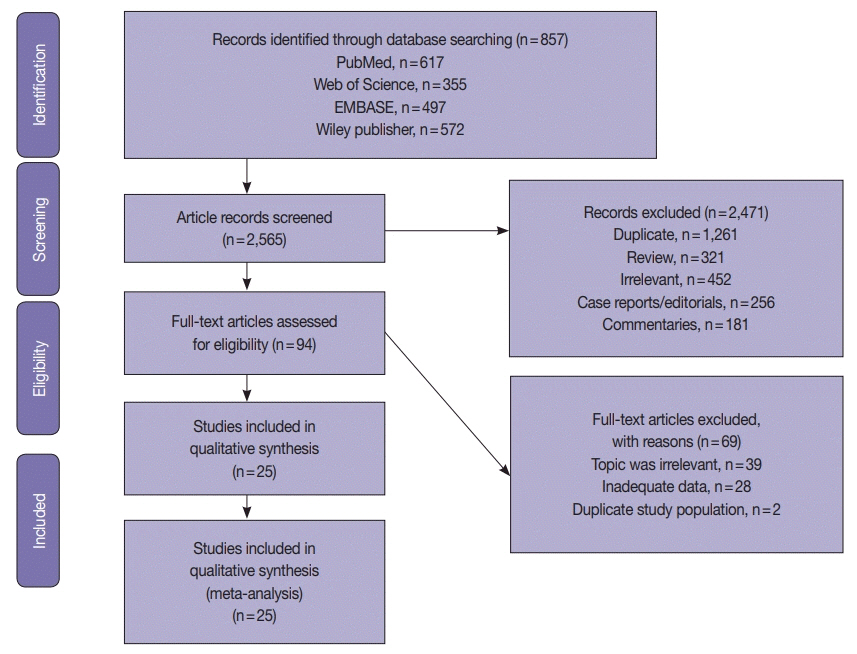
Electronic databases were searched for articles: PubMed, Science Direct, Web of Science, EMBASE, and the Wiley Online Library. Data were extracted from 25 EC studies of D-MMR to generate a clinical dataset of 7,459 patients. A random-effects model produced pooled estimates of D-MMR EC frequency with 95% confidence interval (CI) for meta-analysis.
Results
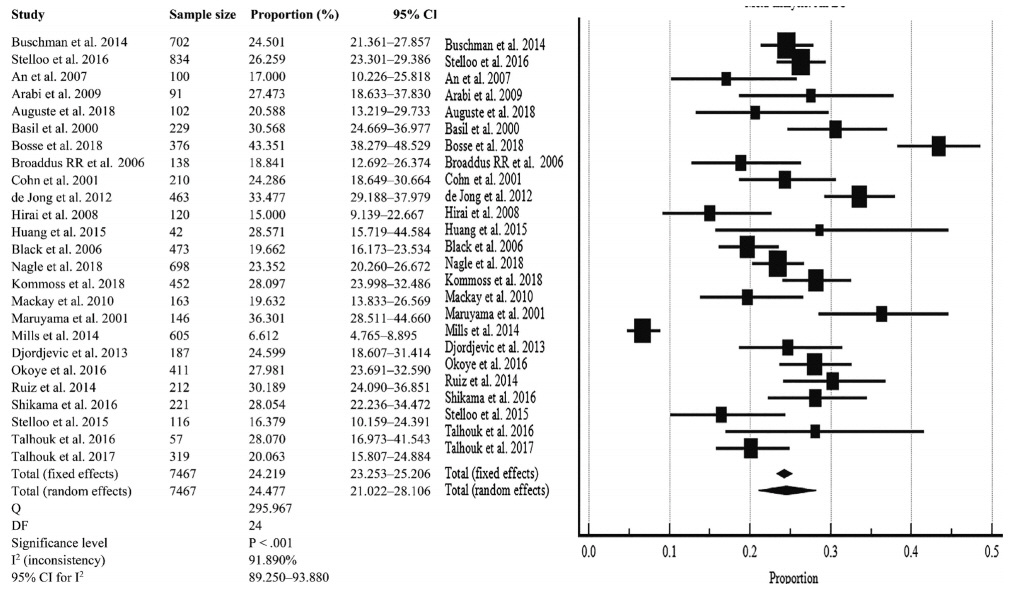
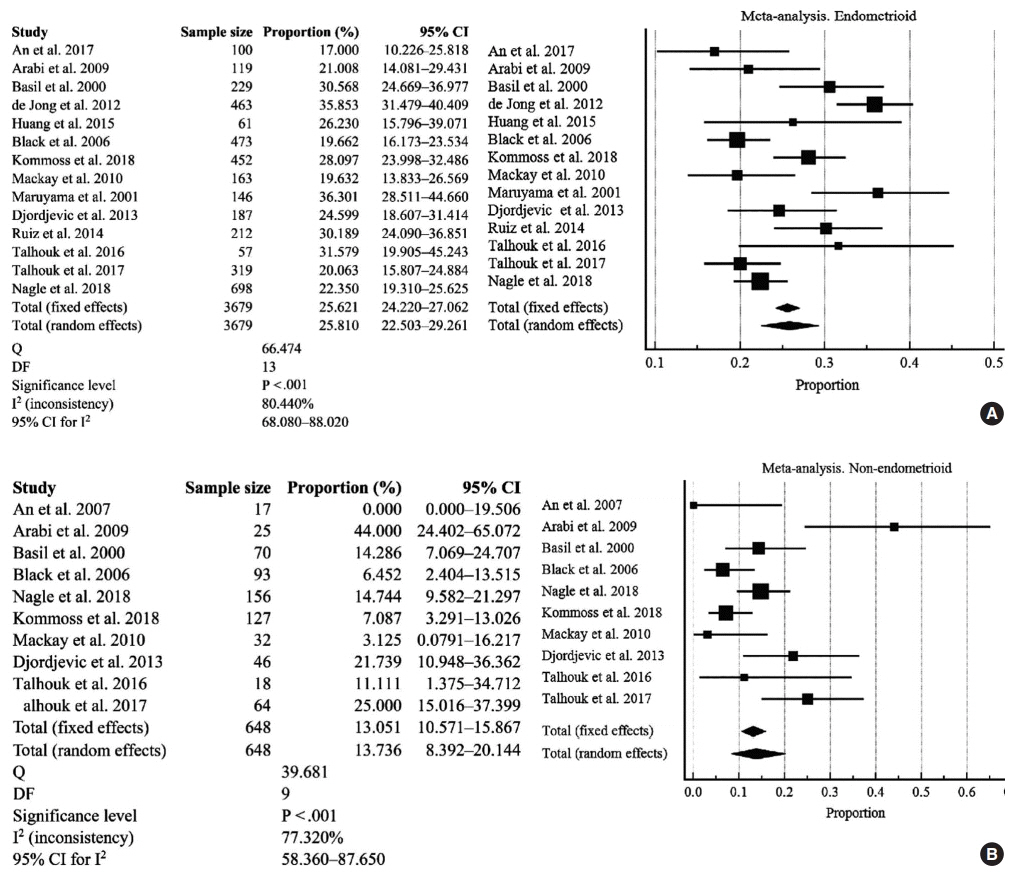
The overall pooled proportion of D-MMR was 24.477% (95% CI, 21.022 to 28.106) in EC. The Lynch syndrome subgroup had 22.907% pooled D-MMR (95% CI, 14.852 to 32.116). D-MMR was highest in type I EC (25.810) (95% CI, 22.503 to 29.261) compared to type II (13.736) (95% CI, 8.392 to 20.144). Pooled D-MMR was highest at EC stage and grades I–II (79.430% and 65.718%, respectively) and lowest in stages III–IV and grade III (20.168% and 21.529%). The pooled odd ratios comparing D-MMR to proficient MMR favored low-stage EC disease (1.565; 0.894 to 2.740), lymphovascular invasion (1.765; 1.293 to 2.409), and myometrial invasion >50% (1.271; 0.871 to 1.853).
Conclusions
Almost one-quarter of EC patients present with D-MMR tumors. The majority has less aggressive endometrioid histology. D-MMR presents at lower tumor stages compared to MMR-proficient cases in EC. However other metastatic parameters are comparatively higher in the D-MMR disease setting.
Figure
Reference
-
References
1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017; 67:7–30.
Article2. Cancer Genome Atlas Research Network; Kandoth C, Schultz N, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013; 497:67–73.
Article3. Church DN, Stelloo E, Nout RA, et al. Prognostic significance of POLE proofreading mutations in endometrial cancer. J Natl Cancer Inst. 2015; 107:402.
Article4. Bell DW, Ellenson LH. Molecular genetics of endometrial carcinoma. Annu Rev Pathol. 2019; 14:339–67.
Article5. Nojadeh JN, Behrouz Sharif S, Sakhinia E. Microsatellite instability in colorectal cancer. EXCLI J. 2018; 17:159–68.6. Buchanan DD, Tan YY, Walsh MD, et al. Tumor mismatch repair immunohistochemistry and DNA MLH1 methylation testing of patients with endometrial cancer diagnosed at age younger than 60 years optimizes triage for population-level germline mismatch repair gene mutation testing. J Clin Oncol. 2014; 32:90–100.
Article7. Stelloo E, Nout RA, Osse EM, et al. Improved risk assessment by integrating molecular and clinicopathological factors in early-stage endometrial cancer-combined analysis of the PORTEC cohorts. Clin Cancer Res. 2016; 22:4215–24.
Article8. An HJ, Kim KI, Kim JY, et al. Microsatellite instability in endometrioid type endometrial adenocarcinoma is associated with poor prognostic indicators. Am J Surg Pathol. 2007; 31:846–53.
Article9. Arabi H, Guan H, Kumar S, et al. Impact of microsatellite instability (MSI) on survival in high grade endometrial carcinoma. Gynecol Oncol. 2009; 113:153–8.
Article10. Auguste A, Genestie C, De Bruyn M, et al. Refinement of high-risk endometrial cancer classification using DNA damage response biomarkers: a TransPORTEC initiative. Mod Pathol. 2018; 31:1851–61.
Article11. Basil JB, Goodfellow PJ, Rader JS, Mutch DG, Herzog TJ. Clinical significance of microsatellite instability in endometrial carcinoma. Cancer. 2000; 89:1758–64.
Article12. Bosse T, Nout RA, McAlpine JN, et al. Molecular classification of grade 3 endometrioid endometrial cancers identifies distinct prognostic subgroups. Am J Surg Pathol. 2018; 42:561–8.
Article13. Broaddus RR, Lynch HT, Chen LM, et al. Pathologic features of endometrial carcinoma associated with HNPCC: a comparison with sporadic endometrial carcinoma. Cancer. 2006; 106:87–94.14. Cohn DE, Mutch DG, Herzog TJ, et al. Genotypic and phenotypic progression in endometrial tumorigenesis: determining when defects in DNA mismatch repair and KRAS2 occur. Genes Chromosomes Cancer. 2001; 32:295–301.15. de Jong RA, Boerma A, Boezen HM, Mourits MJ, Hollema H, Nijman HW. Loss of HLA class I and mismatch repair protein expression in sporadic endometrioid endometrial carcinomas. Int J Cancer. 2012; 131:1828–36.
Article16. Hirai Y, Banno K, Suzuki M, et al. Molecular epidemiological and mutational analysis of DNA mismatch repair (MMR) genes in endometrial cancer patients with HNPCC-associated familial predisposition to cancer. Cancer Sci. 2008; 99:1715–9.
Article17. Huang HN, Lin MC, Tseng LH, et al. Ovarian and endometrial endometrioid adenocarcinomas have distinct profiles of microsatellite instability, PTEN expression, and ARID1A expression. Histopathology. 2015; 66:517–28.
Article18. Black D, Soslow RA, Levine DA, et al. Clinicopathologic significance of defective DNA mismatch repair in endometrial carcinoma. J Clin Oncol. 2006; 24:1745–53.
Article19. Nagle CM, O'Mara TA, Tan Y, et al. Endometrial cancer risk and survival by tumor MMR status. J Gynecol Oncol. 2018; 29:e39.
Article20. Kommoss S, McConechy MK, Kommoss F, et al. Final validation of the ProMisE molecular classifier for endometrial carcinoma in a large population-based case series. Ann Oncol. 2018; 29:1180–8.
Article21. Mackay HJ, Gallinger S, Tsao MS, et al. Prognostic value of microsatellite instability (MSI) and PTEN expression in women with endometrial cancer: results from studies of the NCIC Clinical Trials Group (NCIC CTG). Eur J Cancer. 2010; 46:1365–73.
Article22. Maruyama A, Miyamoto S, Saito T, Kondo H, Baba H, Tsukamoto N. Clinicopathologic and familial characteristics of endometrial carcinoma with multiple primary carcinomas in relation to the loss of protein expression of MSH2 and MLH1. Cancer. 2001; 91:2056–64.
Article23. Mills AM, Liou S, Ford JM, Berek JS, Pai RK, Longacre TA. Lynch syndrome screening should be considered for all patients with newly diagnosed endometrial cancer. Am J Surg Pathol. 2014; 38:1501–9.
Article24. Djordjevic B, Barkoh BA, Luthra R, Broaddus RR. Relationship between PTEN, DNA mismatch repair, and tumor histotype in endometrial carcinoma: retained positive expression of PTEN preferentially identifies sporadic non-endometrioid carcinomas. Mod Pathol. 2013; 26:1401–12.
Article25. Okoye EI, Bruegl AS, Fellman B, Luthra R, Broaddus RR. Defective DNA mismatch repair influences expression of endometrial carcinoma biomarkers. Int J Gynecol Pathol. 2016; 35:8–15.
Article26. Ruiz I, Martin-Arruti M, Lopez-Lopez E, Garcia-Orad A. Lack of association between deficient mismatch repair expression and outcome in endometrial carcinomas of the endometrioid type. Gynecol Oncol. 2014; 134:20–3.
Article27. Shikama A, Minaguchi T, Matsumoto K, et al. Clinicopathologic implications of DNA mismatch repair status in endometrial carcinomas. Gynecol Oncol. 2016; 140:226–33.
Article28. Stelloo E, Bosse T, Nout RA, et al. Refining prognosis and identifying targetable pathways for high-risk endometrial cancer: a TransPORTEC initiative. Mod Pathol. 2015; 28:836–44.
Article29. Talhouk A, Hoang LN, McConechy MK, et al. Molecular classification of endometrial carcinoma on diagnostic specimens is highly concordant with final hysterectomy: earlier prognostic information to guide treatment. Gynecol Oncol. 2016; 143:46–53.
Article30. Talhouk A, McConechy MK, Leung S, et al. Confirmation of ProMisE: a simple, genomics-based clinical classifier for endometrial cancer. Cancer. 2017; 123:802–13.
Article31. Jumaah AS, Salim MM, Al-Haddad HS, McAllister KA, Yasseen AA. The frequency of POLE-mutation in endometrial carcinoma and prognostic implications: a systemic review and meta-analysis. J Pathol Transl Med. 2020; 54:471–9.
Article32. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015; 4:1.
Article33. Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011; 155:529–36.
Article34. Kumar V, Abbas AK, Aster JC. Robbins and Contran pathologic basis of disease. 9th ed. Philadelphia: Saunders-Elsevier;2015. p. 1014–8.35. MedCalc. Statistical software version 15.8 [Internet]. Ostend: MedCalc Software bvba;2015. [cited 2020 Dec 10]. Available from: https://www.medcalc.org.36. Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ. 2011; 342:d549.
Article37. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 327:557–60.
Article38. Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011; 343:d4002.
Article39. Ryan NA, Glaire MA, Blake D, Cabrera-Dandy M, Evans DG, Crosbie EJ. The proportion of endometrial cancers associated with Lynch syndrome: a systematic review of the literature and metaanalysis. Genet Med. 2019; 21:2167–80.
Article40. Lorenzi M, Amonkar M, Zhang J, Mehta S, Law KL. Epidemiology of microsatellite instability high (MSI-H) and deficient mismatch repair (dMMR) in solid tumors: a structured literature review. J Oncol. 2020; 2020:1807929.
Article41. Ryan NAJ, Blake D, Cabrera-Dandy M, Glaire MA, Evans DG, Crosbie EJ. The prevalence of Lynch syndrome in women with endometrial cancer: a systematic review protocol. Syst Rev. 2018; 7:121.
Article42. Jerzak KJ, Duska L, MacKay HJ. Endocrine therapy in endometrial cancer: an old dog with new tricks. Gynecol Oncol. 2019; 153:175–83.
Article43. Ashley CW, Da Cruz Paula A, Kumar R, et al. Analysis of mutational signatures in primary and metastatic endometrial cancer reveals distinct patterns of DNA repair defects and shifts during tumor progression. Gynecol Oncol. 2019; 152:11–9.
Article44. Sargent DJ, Marsoni S, Monges G, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol. 2010; 28:3219–26.
Article45. Fujiyoshi K, Yamamoto G, Takenoya T, et al. Metastatic pattern of stage IV colorectal cancer with high-frequency microsatellite instability as a prognostic factor. Anticancer Res. 2017; 37:239–47.
Article46. Fountzilas E, Kotoula V, Pentheroudakis G, et al. Prognostic implications of mismatch repair deficiency in patients with nonmetastatic colorectal and endometrial cancer. ESMO Open. 2019; 4:e000474.
Article47. Copija A, Waniczek D, Witkos A, Walkiewicz K, Nowakowska-Zajdel E. Clinical significance and prognostic relevance of microsatellite instability in sporadic colorectal cancer patients. Int J Mol Sci. 2017; 18:107.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- DNA mismatch repair-related protein loss as a prognostic factor in endometrial cancers
- Elucidation of genomic origin of synchronous endometrial and ovarian cancer (SEO) by genomic and microsatellite analysis
- Clinical significance of mismatch repair genes immunohistochemical expression of complex endometrial hyperplasia
- Identification of Target Genes in the Endometrial Carcinomas with Microsatellite Mutator Phenotype
- An Introduction of the Systematic Review and Meta-Analysis