Brain Tumor Res Treat.
2021 Apr;9(1):1-8. 10.14791/btrt.2021.9.e8.
The Korean Society for Neuro-Oncology (KSNO) Guideline for Adult Diffuse Midline Glioma: Version 2021.1
- Affiliations
-
- 1Department of Radiation Oncology, Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, Korea
- 2Department of Radiation Oncology, SMG-SNU Boramae Medical Center, Seoul, Korea
- 3Division of Neurooncology and Department of Neurosurgery, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon, Korea
- 4Department of Neurosurgery, Yeungnam University Hospital, Yeungnam University College of Medicine, Daegu, Korea
- 5Department of Radiation Oncology, CHA Bundang Medical Center, CHA University School of Medicine, Seongnam, Korea
- 6Department of Neurosurgery, Chungbuk National University Hospital, Chungbuk National University College of Medicine, Cheongju, Korea
- 7Department of Neurosurgery, Chungnam National University Hospital, Chungnam National University School of Medicine, Daejeon, Korea;
- 8Department of Neurosurgery, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea
- 9Department of Neurosurgery, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 10Department of Radiation Oncology, Yongin Severance Hospital, Yonsei University College of Medicine, Yongin, Korea
- 11Department of Neurosurgery, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- 12Department of Genomic Medicine, Department of Neurology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- 13Department of Neurosurgery, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- 14Department of Radiology and Research Institute of Radiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 15Department of Neurosurgery, Catholic Kwandong University, International St. Mary’s Hospital, Incheon, Korea
- 16Department of Neurosurgery, Incheon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 17Department of Pathology, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- 18Department of Neurosurgery, St. Vincent’s Hospital, College of Medicine, The Catholic University of Korea, Suwon, Korea
- 19Department of Neurosurgery, Dong-A University Hospital, Dong-A University College of Medicine, Busan, Korea
- 20Department of Radiation Oncology, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 21Department of Neurosurgery, Uijeongbu St. Mary’s Hospital, The Catholic University of Korea, Uijeongbu, Korea
- 22Department of Neurosurgery, Hanyang University Guri Hospital, Hanyang University College of Medicine, Guri, Korea
- 23Division of Hematology/Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 24Department of Neurosurgery, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- 25Department of Radiation Oncology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 26Department of Hospital Pathology, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 27Department of Cancer Control, Graduate School of Cancer Science and Policy, National Cancer Center, Goyang, Korea
- KMID: 2515075
- DOI: http://doi.org/10.14791/btrt.2021.9.e8
Abstract
- Background
There have been no guidelines for the management of adult patients with diffuse midline glioma (DMG), H3K27M-mutant in Korea since the 2016 revised WHO classification newly defined this disease entity. Thus, the Korean Society for Neuro-Oncology (KSNO), a multidisciplinary academic society, had begun preparing guidelines for DMG since 2019.
Methods
The Working Group was composed of 27 multidisciplinary medical experts in Korea. References were identified through searches of PubMed, MEDLINE, EMBASE, and Cochrane CENTRAL using specific and sensitive keywords as well as combinations of keywords. As ‘diffuse midline glioma’ was recently defined, and there was no international guideline, trials and guidelines of ‘diffuse intrinsic pontine glioma’ or ‘brain stem glioma’ were thoroughly reviewed first.
Results
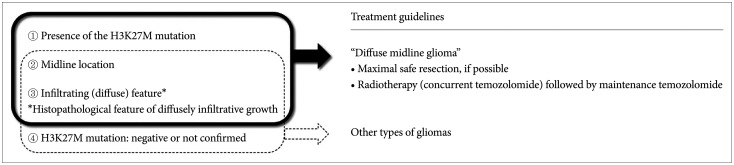
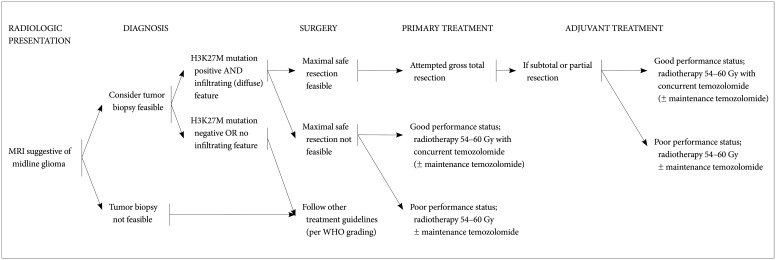
The core contents are as follows. The DMG can be diagnosed when all of the following three criteria are satisfied: the presence of the H3K27M mutation, midline location, and infiltrating feature. Without identification of H3K27M mutation by diagnostic biopsy, DMG cannot be diagnosed. For the primary treatment, maximal safe resection should be considered for tumors when feasible. Radiotherapy is the primary option for tumors in case the total resection is not possible. A total dose of 54 Gy to 60 Gy with conventional fractionation prescribed at 1-2 cm plus gross tumor volume is recommended. Although no chemotherapy has proven to be effective in DMG, concurrent chemoradiotherapy (± maintenance chemotherapy) with temozolomide following WHO grade IV glioblastoma’s protocol is recommended.
Conclusion
The detection of H3K27M mutation is the most important diagnostic criteria for DMG. Combination of surgery (if amenable to surgery), radiotherapy, and chemotherapy based on comprehensive multidisciplinary discussion can be considered as the treatment options for DMG.
Figure
Cited by 1 articles
-
Korean Brain Tumor Society Consensus Review for the Practical Recommendations on Glioma Management in Korea
Chul-Kee Park, Jong Hee Chang
J Korean Neurosurg Soc. 2023;66(3):308-315. doi: 10.3340/jkns.2023.0046.
Reference
-
1. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK. WHO classification of tumours of the central nervous system. Revised 4th ed. Lyon: International Agency for Research on Cancer;2016.2. Karremann M, Gielen GH, Hoffmann M, et al. Diffuse high-grade gliomas with H3 K27M mutations carry a dismal prognosis independent of tumor location. Neuro Oncol. 2018; 20:123–131. PMID: 29016894.3. Schreck KC, Ranjan S, Skorupan N, et al. Incidence and clinicopathologic features of H3 K27M mutations in adults with radiographically-determined midline gliomas. J Neurooncol. 2019; 143:87–93. PMID: 30864101.4. Wu G, Broniscer A, McEachron TA, et al. Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat Genet. 2012; 44:251–253. PMID: 22286216.5. Nikbakht H, Panditharatna E, Mikael LG, et al. Spatial and temporal homogeneity of driver mutations in diffuse intrinsic pontine glioma. Nat Commun. 2016; 7:11185. PMID: 27048880.6. Hoffman LM, DeWire M, Ryall S, et al. Spatial genomic heterogeneity in diffuse intrinsic pontine and midline high-grade glioma: implications for diagnostic biopsy and targeted therapeutics. Acta Neuropathol Commun. 2016; 4:1. PMID: 26727948.7. Louis DN, Giannini C, Capper D, et al. cIMPACT-NOW update 2: diagnostic clarifications for diffuse midline glioma, H3 K27M-mutant and diffuse astrocytoma/anaplastic astrocytoma, IDH-mutant. Acta Neuropathol. 2018; 135:639–642. PMID: 29497819.8. Khuong-Quang DA, Buczkowicz P, Rakopoulos P, et al. K27M mutation in histone H3.3 defines clinically and biologically distinct subgroups of pediatric diffuse intrinsic pontine gliomas. Acta Neuropathol. 2012; 124:439–447. PMID: 22661320.9. Meyronet D, Esteban-Mader M, Bonnet C, et al. Characteristics of H3 K27M-mutant gliomas in adults. Neuro Oncol. 2017; 19:1127–1134. PMID: 28201752.10. Walker DA, Liu J, Kieran M, et al. A multi-disciplinary consensus statement concerning surgical approaches to low-grade, high-grade astrocytomas and diffuse intrinsic pontine gliomas in childhood (CPN Paris 2011) using the Delphi method. Neuro Oncol. 2013; 15:462–468. PMID: 23502427.11. Epstein F, Constantini S. Practical decisions in the treatment of pediatric brain stem tumors. Pediatr Neurosurg. 1996; 24:24–34. PMID: 8817612.12. Qiu T, Chanchotisatien A, Qin Z, et al. Imaging characteristics of adult H3 K27M-mutant gliomas. J Neurosurg. 2020; 133:1662–1670.13. Aboian MS, Solomon DA, Felton E, et al. Imaging characteristics of pediatric diffuse midline gliomas with histone H3 K27M mutation. AJNR Am J Neuroradiol. 2017; 38:795–800. PMID: 28183840.14. El-Khouly FE, Veldhuijzen van, Santa-Maria Lopez V, et al. Diagnostics and treatment of diffuse intrinsic pontine glioma: where do we stand? J Neurooncol. 2019; 145:177–184. PMID: 31522324.15. Weller M, van den Bent M, Preusser M, et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol. 2021; 18:170–186. PMID: 33293629.16. Sievers P, Sill M, Schrimpf D, et al. A subset of pediatric-type thalamic gliomas share a distinct DNA methylation profile, H3K27me3 loss and frequent alteration of EGFR. Neuro Oncol. 2021; 23:34–43. PMID: 33130881.17. Weller M, van den Bent M, Tonn JC, et al. European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol. 2017; 18:e315–e329. PMID: 28483413.18. Niu X, Wang T, Zhou X, et al. Surgical treatment and survival outcome of patients with adult thalamic glioma: a single institution experience of 8 years. J Neurooncol. 2020; 147:377–386. PMID: 32157551.19. Himes BT, Zhang L, Daniels DJ. Treatment strategies in diffuse midline gliomas with the H3K27M mutation: the role of convection-enhanced delivery in overcoming anatomic challenges. Front Oncol. 2019; 9:31. PMID: 30800634.20. Cinalli G, Aguirre DT, Mirone G, et al. Surgical treatment of thalamic tumors in children. J Neurosurg Pediatr. 2018; 21:247–257. PMID: 29271729.21. Hargrave D, Bartels U, Bouffet E. Diffuse brainstem glioma in children: critical review of clinical trials. Lancet Oncol. 2006; 7:241–248. PMID: 16510333.22. Korones DN, Fisher PG, Kretschmar C, et al. Treatment of children with diffuse intrinsic brain stem glioma with radiotherapy, vincristine and oral VP-16: a Children's Oncology Group phase II study. Pediatr Blood Cancer. 2008; 50:227–230. PMID: 17278121.23. Massimino M, Spreafico F, Biassoni V, et al. Diffuse pontine gliomas in children: changing strategies, changing results? A mono-institutional 20-year experience. J Neurooncol. 2008; 87:355–361. PMID: 18217208.24. Gallitto M, Lazarev S, Wasserman I, et al. Role of radiation therapy in the management of diffuse intrinsic pontine glioma: a systematic review. Adv Radiat Oncol. 2019; 4:520–531. PMID: 31360809.25. Combs SE, Steck I, Schulz-Ertner D, et al. Long-term outcome of highprecision radiotherapy in patients with brain stem gliomas: results from a difficult-to-treat patient population using fractionated stereotactic radiotherapy. Radiother Oncol. 2009; 91:60–66. PMID: 19285356.26. Muroi A, Mizumoto M, Ishikawa E, et al. Proton therapy for newly diagnosed pediatric diffuse intrinsic pontine glioma. Childs Nerv Syst. 2020; 36:507–512. PMID: 31728705.27. Mandell LR, Kadota R, Freeman C, et al. There is no role for hyperfractionated radiotherapy in the management of children with newly diagnosed diffuse intrinsic brainstem tumors: results of a Pediatric Oncology Group phase III trial comparing conventional vs. hyperfractionated radiotherapy. Int J Radiat Oncol Biol Phys. 1999; 43:959–964. PMID: 10192340.28. Freeman CR, Krischer JP, Sanford RA, et al. Final results of a study of escalating doses of hyperfractionated radiotherapy in brain stem tumors in children: a Pediatric Oncology Group study. Int J Radiat Oncol Biol Phys. 1993; 27:197–206. PMID: 8407392.29. Freeman CR, Krischer J, Sanford RA, Burger PC, Cohen M, Norris D. Hyperfractionated radiotherapy in brain stem tumors: results of a Pediatric Oncology Group study. Int J Radiat Oncol Biol Phys. 1988; 15:311–318. PMID: 2841262.30. Freeman CR, Krischer J, Sanford RA, et al. Results of treatment at the 7020 cGy dose level of Pediatric Oncology Group study #8495. Cancer. 1991; 68:474–481. PMID: 2065266.31. Allen J, Siffert J, Donahue B, et al. A phase I/II study of carboplatin combined with hyperfractionated radiotherapy for brainstem gliomas. Cancer. 1999; 86:1064–1069. PMID: 10491535.32. Packer RJ, Boyett JM, Zimmerman RA, et al. Hyperfractionated radiation therapy (72 Gy) for children with brain stem gliomas. A Childrens Cancer Group Phase I/II Trial. Cancer. 1993; 72:1414–1421. PMID: 8339232.33. Packer RJ, Boyett JM, Zimmerman RA, et al. Outcome of children with brain stem gliomas after treatment with 7800 cGy of hyperfractionated radiotherapy. A Childrens Cancer Group Phase I/II Trial. Cancer. 1994; 74:1827–1834. PMID: 8082086.34. Walter AW, Gajjar A, Ochs JS, et al. Carboplatin and etoposide with hyperfractionated radiotherapy in children with newly diagnosed diffuse pontine gliomas: a phase I/II study. Med Pediatr Oncol. 1998; 30:28–33. PMID: 9371386.35. Marcus KJ, Dutton SC, Barnes P, et al. A phase I trial of etanidazole and hyperfractionated radiotherapy in children with diffuse brainstem glioma. Int J Radiat Oncol Biol Phys. 2003; 55:1182–1185. PMID: 12654425.36. Zaghloul MS, Eldebawy E, Ahmed S, et al. Hypofractionated conformal radiotherapy for pediatric diffuse intrinsic pontine glioma (DIPG): a randomized controlled trial. Radiother Oncol. 2014; 111:35–40. PMID: 24560760.37. Janssens GO, Jansen MH, Lauwers SJ, et al. Hypofractionation vs conventional radiation therapy for newly diagnosed diffuse intrinsic pontine glioma: a matched-cohort analysis. Int J Radiat Oncol Biol Phys. 2013; 85:315–320. PMID: 22682807.38. Izzuddeen Y, Gupta S, Haresh KP, Sharma D, Giridhar P, Rath GK. Hypofractionated radiotherapy with temozolomide in diffuse intrinsic pontine gliomas: a randomized controlled trial. J Neurooncol. 2020; 146:91–95. PMID: 31728883.39. Hayashi A, Ito E, Omura M, et al. Hypofractionated radiotherapy in children with diffuse intrinsic pontine glioma. Pediatr Int. 2020; 62:47–51. PMID: 31785177.40. Negretti L, Bouchireb K, Levy-Piedbois C, et al. Hypofractionated radiotherapy in the treatment of diffuse intrinsic pontine glioma in children: a single institution's experience. J Neurooncol. 2011; 104:773–777. PMID: 21327862.41. Zaghloul MS, Akoush H, Ahmed S, et al. Hypofractionated radiation for pediatric diffuse intrinsic pontine glioma (DIPG): younger children have better survival. Int J Radiat Oncol Biol Phys. 2018; 101:1008–1009.42. Fontanilla HP, Pinnix CC, Ketonen LM, et al. Palliative reirradiation for progressive diffuse intrinsic pontine glioma. Am J Clin Oncol. 2012; 35:51–57. PMID: 21297433.43. Lassaletta A, Strother D, Laperriere N, et al. Reirradiation in patients with diffuse intrinsic pontine gliomas: the Canadian experience. Pediatr Blood Cancer. 2018; 65:e26988. PMID: 29369515.44. Amsbaugh MJ, Mahajan A, Thall PF, et al. A Phase 1/2 Trial of Reirradiation for Diffuse Intrinsic Pontine Glioma. Int J Radiat Oncol Biol Phys. 2019; 104:144–148. PMID: 30610915.45. Janssens GO, Gandola L, Bolle S, et al. Survival benefit for patients with diffuse intrinsic pontine glioma (DIPG) undergoing re-irradiation at first progression: a matched-cohort analysis on behalf of the SIOP-EHGG/ DIPG working group. Eur J Cancer. 2017; 73:38–47. PMID: 28161497.46. Kline C, Liu SJ, Duriseti S, et al. Reirradiation and PD-1 inhibition with nivolumab for the treatment of recurrent diffuse intrinsic pontine glioma: a single-institution experience. J Neurooncol. 2018; 140:629–638. PMID: 30206764.47. Massimino M, Biassoni V, Miceli R, et al. Results of nimotuzumab and vinorelbine, radiation and re-irradiation for diffuse pontine glioma in childhood. J Neurooncol. 2014; 118:305–312. PMID: 24696052.48. Jennings MT, Sposto R, Boyett JM, et al. Preradiation chemotherapy in primary high-risk brainstem tumors: phase II study CCG-9941 of the Children's Cancer Group. J Clin Oncol. 2002; 20:3431–3437. PMID: 12177103.49. Finlay JL, August C, Packer R, et al. High-dose multi-agent chemotherapy followed by bone marrow ‘rescue’ for malignant astrocytomas of childhood and adolescence. J Neurooncol. 1990; 9:239–248. PMID: 1964962.50. Bouffet E, Raquin M, Doz F, et al. Radiotherapy followed by high dose busulfan and thiotepa: a prospective assessment of high dose chemotherapy in children with diffuse pontine gliomas. Cancer. 2000; 88:685–692. PMID: 10649264.51. Dunkel IJ, O'Malley B, Finlay JL. Is there a role for high-dose chemotherapy with stem cell rescue for brain stem tumors of childhood? Pediatr Neurosurg. 1996; 24:263–266. PMID: 8933570.52. Cohen KJ, Heideman RL, Zhou T, et al. Temozolomide in the treatment of children with newly diagnosed diffuse intrinsic pontine gliomas: a report from the Children's Oncology Group. Neuro Oncol. 2011; 13:410–416. PMID: 21345842.53. Jalali R, Raut N, Arora B, et al. Prospective evaluation of radiotherapy with concurrent and adjuvant temozolomide in children with newly diagnosed diffuse intrinsic pontine glioma. Int J Radiat Oncol Biol Phys. 2010; 77:113–118. PMID: 19647954.54. Sharp JR, Bouffet E, Stempak D, et al. A multi-centre Canadian pilot study of metronomic temozolomide combined with radiotherapy for newly diagnosed paediatric brainstem glioma. Eur J Cancer. 2010; 46:3271–3279. PMID: 20656474.55. Bailey S, Howman A, Wheatley K, et al. Diffuse intrinsic pontine glioma treated with prolonged temozolomide and radiotherapy--results of a United Kingdom phase II trial (CNS 2007 04). Eur J Cancer. 2013; 49:3856–3862. PMID: 24011536.56. Pollack IF, Stewart CF, Kocak M, et al. A phase II study of gefitinib and irradiation in children with newly diagnosed brainstem gliomas: a report from the Pediatric Brain Tumor Consortium. Neuro Oncol. 2011; 13:290–297. PMID: 21292687.57. Gilbertson RJ, Hill DA, Hernan R, et al. ERBB1 is amplified and overexpressed in high-grade diffusely infiltrative pediatric brain stem glioma. Clin Cancer Res. 2003; 9(10 Pt 1):3620–3624. PMID: 14506149.58. Chi AS, Tarapore RS, Hall MD, et al. Pediatric and adult H3 K27Mmutant diffuse midline glioma treated with the selective DRD2 antagonist ONC201. J Neurooncol. 2019; 145:97–105. PMID: 31456142.59. Cooney T, Onar-Thomas A, Huang J, et al. DIPG-22. A phase 1 trial of the histone deacetylase inhibitor panobinostat in pediatric patients with recurrent or refractory diffuse intrinsic pontine glioma: a Pediatric Brain Tumor Consortium (PBTC) study. Neuro Oncol. 2018; 20(Issue suppl_2):i53.60. Lin GL, Wilson KM, Ceribelli M, et al. Therapeutic strategies for diffuse midline glioma from high-throughput combination drug screening. Sci Transl Med. 2019; 11:eaaw0064. PMID: 31748226.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Overview of Practical Guidelines for Gliomas by KSNO, NCCN, and EANO
- A National Consensus Survey for Current Practicein Brain Tumor Management II:Diffuse Midline Glioma and Meningioma
- The Korean Society for Neuro-Oncology (KSNO) Guideline for WHO Grade II Cerebral Gliomas in Adults: Version 2019.01
- Yesterdays, Todays, and Tomorrows—Korean Society for Pediatric Neuro-Oncology*
- The Korean Society for Neuro-Oncology (KSNO) Guideline for WHO Grade III Cerebral Gliomas in Adults: Version 2019.01